Introduction
Spirulina platensis (SP) is a blue-green alga having diverse biological activity. Early, due to high content of highly valuable proteins, indispensable amino acids, vitamins, beta-carotene and other pigments, mineral substances, indispensable fatty acids and polysaccharides, SP has been found suitable for use as bioactive additive.1 Recent attention has been given to immune-stimulant role of SP.2
In 1994, the first report for immune-modulatory effect of this alga on mice through enhanced IL-1 antibody production.3 Chicken diets were contained less than 1% Spirulina lead to significantly enhance the defense systems for increased microbial killing, antigen processing and greater T-cell activity. Subsequently, more researches were investigated the role of Spirulina to enhance immune system in different animal models as dogs and cats as well as human.4 Newcastle disease virus (NDV) stands behind most of mortalities and morbidities in poultry.5 The infection control depends mainly on vaccination of chickens with the commercial inactivated NDV vaccine. Many of these vaccine batches are submitted annually to the Central Laboratory for Evaluation of Veterinary Biologics (CLEVB), Cairo, Egypt for reviewing its quality. Potency of this type of vaccine is evaluated routinely by vaccination-challenge test (efficacy) in susceptible SPF chickens following the protocols.6
In this study, protective efficacy of an inactivated Newcastle vaccine (Ulster 2 strain) is determined in SPF chickens that were supplied with different concentrations of SP (0.5, 1, 1.5 and 2 g/kg ration) in comparison with SPF chickens fed with the same ration without any supplement against heterologous circulating NDV genotype VII.
Material and Methods
Preparation of Spirulina extract: A crude extract was prepared from SP using a patent-pending procedure. Raw material was extracted two times with 50% ethanol at 70°C, 45 min each time. Supernatants from both extractions were combined following centrifugation for 5 min at 1500 g. The ethanol concentration of the extract was adjusted to 75% by addition of 1 volume of cold ethanol. Following incubation for several hours at -20°C, perceptible material was collected by centrifugation at 1500 g and subsequently washed with cold ethanol. The final extract material was dried and represented a 15% yield of raw material dry weight.
Experimental Chicken: SPF chickens were obtained from Khom Oshem farm, El Fayoum as one day old. They were reared and housed in positive pressure stainless steel isolation cabinets with continuous light exposure.
Vaccine: The commercial inactivated NDV vaccine contained (Ulster2C strain) which was used for vaccination of SPF chickens with one field dose recommended for poultry that was administrated intramuscularly at a dose of 0.3mL/bird. The batch No. is (L469199) and it's expired by 23/11/2021.
Serum samples: Blood samples were collected from jugular vein of ten vaccinated SPF chickens from each group and sera were separated to conduct Hemagglutination inhibition test (HI test).
Virus: Local NDV genotype VII was obtained from Strain Bank of Central Laboratory for Evaluation of Veterinary Biologics (CLEVB) which has (accession no. KM288609) to be used as challenge virus and as heterologous NDV antigen with a titer of 8 log 2 HA units/mL and used at a final concentration of 4 HA/mL in HI test for the tested serum samples.
Propagation and titration of NDV genotype VII: It was carried out according to the manual of the World Organization of Animal Health.7
Calculation of Egg infective dose/50 (EID50) for local NDV genotype VII: It was done according to the manual of the World Organization of Animal Health.7
Serological tests: Hemagglutination (HA) and HI assays were performed using the standard microtiter plate method as recommended.7 The HI tests was carried out with 4 HA units of NDV genotype VII per well.
Measurement of the protection efficacy %: By using challenge test through inoculation of 106 EID50/SPF chickens of NDV genotype VII intramuscularly at a dose 0.5mL/bird.7
Measurement of viral shedding by RT-qPCR: Oropharyngeal swabs were taken from all groups of chickens at 3, 5, 7 and 10 days post challenge and prepared then kept at -80°C till use.7
Qrt-PCR: RNA was extracted from swabs using QIAamp Viral RNA Mini Kit that supplied from Qiagen, Valencia, CA, USA, Cat. No. 52906. Samples were amplified using Invitrogen superscript® III platinum® one- step Quantitative RT-PCR Cat. No 11732-088 to investigate the presence or absence of M gene of ND virus following the manufacture instructions using primers and probe and reaction condition.8) The test was conducted in a CFX 96 touch TM Real time PCR (Table 1).
Table 1 Oligonucleotide primers used in RT-PCR for detection of NDV M- protein gene.
| Primer | Sequence (5′ - 3′ ) |
|---|---|
| Forward ND-M+4100 | AGTGATGTGCTCGGACCTTC |
| Reverse ND-M-4220 | CCTGAGGAGAGAGGCATTTGCTA |
| Probe | HEX-TTCTCTAGCAGTGGGACAGCCTGC-BHQ |
Experimental design: A total 120 SPF chickens at 7 days old of age were distributed randomly into three groups. The first group (80 SPF chickens) was allotted into four replicates (20 SPF chickens/replicate) which were supplemented with different SP concentration (0.5, 1, 1.5, and 2 g/kg in ration), then vaccinated with NDV vaccine at 21 days old age. The second group (20 SPF chickens) were fed without any supplement, then vaccinated with the same vaccine at the same age of the previous group despite, the third group control unvaccinated group (20 chickens) without any treatment.
Individual blood samples were collected from ten birds of each group weekly between the first and 4 weeks after inoculation and NDV-HI antibodies were measured in each collected serum sample by HI test. After 28 days post-vaccination, ten birds from all groups were challenged intramuscularly at a dose of 0.5 mL/ bird by 106 EID50 of local NDV genotype VII. Oropharyngeal swabs were taken from all groups of chickens at 3, 5, 7 and 10 days post challenge.
Ethical approval: Institutional Animal Care and use committee at Central Laboratory for Evaluation of veterinary Biologics hereby acknowledge the research manuscript and it has been reviewed under our research authority and is deemed compliance to bioethical standards in good faith.
Statistical Analysis: Data generated from immune responses were subjected to one way analysis of variance (ANOVA). Variant means were separated post hoc using the least significant difference (LSD) method;9 p<0.05 were accepted as significant.
Results
Hemagglutination inhibition test
The data revealed from Table 2, showed low mean HI antibody titers at one week post vaccination (WPV) for all vaccinated groups. Mean HI antibody titer increased gradually till reached its peak at 4th WPV to be 7.6, 8, 8.3, 8.9 and 7.4 log2 in the sera of vaccinated SPF chickens supplemented with 0.5, 1, 1.5 and 2 g SP/kg ration and control treatment group, respectively. Vaccinated SPF chickens were consumed SP algae in ration exhibited higher immune response than those induced by the other group. There were a significant difference at 2nd WPV in all groups consumed 1, 1.5 and 2 g of SP in ration to other vaccinated chickens fed ration without SP as shown in Table 3.
Table 2 Mean ND-HI antibody titer produced by sera of SPF chickens after 1, 2, 3 and 4 weeks post vaccination with one dose of commercial ND vaccine.
| Mean HI antibody titer (log2) | |||||
|---|---|---|---|---|---|
| Group of chickens | Spirulina (g/kg) in ration | 1st WPV | 2nd WPV | 3rd WPV | 4th WPV |
| Vaccinated chickens were supplemented with different SP g/kg in ration | 0.5 g/kg | 1.2 ±1.09280 | 4.3 ±0.67495 | 6.4 ±0.69921 | 7.6 ±0.51640 |
| 1 g/kg | 1.4 ±0.96609 | 5.1 ±0.87560 | 7.3 ±0.67495 | 8 ±0.73786 | |
| 1.5 g/kg | 1.6 ±0.84327 | 5.4 ±0.69921 | 7.4 ±0.51640 | 8.3 ±0.67495 | |
| 2 g/kg | 2 ±0.94281 | 5.9 ±0.56765 | 7.8 ±0.63246 | 8.9 ±0.73786 | |
| Vaccinated chickens without SP supplement in ration | 1.2 ±1.03280 | 4.1 ±0.87560 | 6 ±0.81650 | 7.4 ±0.69921 | |
| Control non-vaccinated chickens | 0 | 0 | 0 | 0 |
Table 3 Significant values between vaccinated chickens without SP supplement in ration to Vaccinated chickens consumed different concentration SP g/kg of ration.
| Significant values | |||||
|---|---|---|---|---|---|
| Vaccinated chickens supplied with different SP g/kg of ration | |||||
| Group | WPV | 0.5 g/kg | 1 g/kg | 1.5 g/kg | 2 g/kg |
| Vaccinated chickens without Spirulina supplement in ration | 1 WPV | 0.961 | 0.553 | 0.179 | 0.513 |
| 2 WPV | 0.649 | 0.005 | 0.000 | 0.000 | |
| 3 WPV | 0.365 | 0.000 | 0.000 | 0.000 | |
| 4 WPV | 0.074 | 0.000 | 0.000 | 0.000 |
Significance difference ≤ 0.005
Protection efficacy
It was found that all vaccinated SPF chickens groups consumed SP in ration with different concentrations (0.5, 1, 1.5 and 2 g/kg ration) were protected the infection against local NDV genotype VII with a different percentage: 90, 100, 100 and 100%, respectively. The group fed without SP has less protection% reached 80% in contrast to the control group that showed 100% mortality (Table 4).
Table 4 Protection % of SPF chickens after 4 weeks post vaccination with one dose of commercial ND vaccine following challenge by local NDV genotype VII.
| Groups | Total mortality | Protection % | |
|---|---|---|---|
| 0.5 g/kg | 1/10 | 90% | |
| Spirulina g/kg in ration for vaccinated chickens | 1 g/kg | 0/10 | 100% |
| 1.5 g/kg | 0/10 | 100% | |
| 2 g/kg | 0/10 | 100% | |
| Vaccinated chickens without Spirulina supplement | 2/10 | 80% | |
| Control non vaccinated | 5/5 | 0% |
Viral shedding
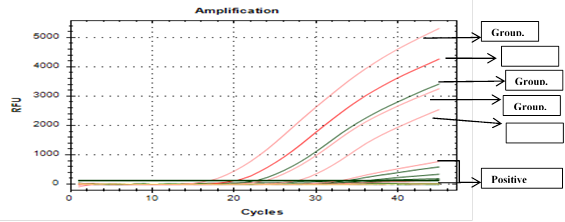
Oropharyngeal swabs were collected from all groups after 3, 5, 7 and 10 days post challenge (DPC) examined by real time RT-PCR to calculate viral Shedding of Local NDV genotype VII. The exhibited data from Table 5 and Figure 1 showed the challenged vaccinated chickens released the virus with low titers in comparison to unvaccinated challenged chickens. The reduction of viral shedding reached 3.95, 4.65, 5.45, 6.25 and 3.3 log10 in challenged vaccinated chicken consumed Spirulina algae 0.5, 1, 1.5 and 2 g/kg of ration and the other challenged vaccinated not treated respectively.
Table 5 Reduction in viral shedding (log10) from SPF chickens after 4 weeks post vaccination with one dose of commercial ND vaccine following challenge by local NDV genotype VII.
| Titer of Shedding Virus titer (log10) | |||||||
|---|---|---|---|---|---|---|---|
| Group of chickens | Spirulina g/kg in ration | 3rd DPC | 5th DPC | 7th DPC | 10th DPC | Mean viral shedding | Reduction in viral shedding |
| Vaccinated chickens were supplemented with different SP g/kg in ration | 0.05% | 4 | 4.1 | 3 | 1.8 | 3.2 | 3.95 (55%) |
| 0.1% | 3.3 | 3.1 | 2.3 | 1.4 | 2.5 | 4.65 (65%) | |
| 0.15% | 2.1 | 2.4 | 1.6 | 1 | 1.7 | 5.45 (76%) | |
| 0.2% | 1 | 1.3 | 0.9 | 0.4 | 0.9 | 6.25 (87%) | |
| Vaccinated chickens without SP supplement in ration | 4.8 | 4.6 | 4 | 2 | 3.85 | 3.3 (46%) | |
| Control non-vaccinated chickens | 7.1 | 7.2 | - | - | 7.15 | 0 | |
Discussion
Spirulina is a cyanobacterium species.10 In poultry, some recent studies have shown that feeding SP is responsible for improvement of immune functions, subsequently increased disease resistance, improved survival and growth rates.3
In this study, the immunostimulant effect of Spirulina was assessed through vaccination of SPF chickens with commercial inactivated NDV vaccine which clustered into five groups (20 SPF chickens/group). Five groups were supplied with different SP grams (0.5, 1, 1.5 and 2) per kg of ration respectively, in comparison with control treatment group which agreed with some researchers.11 Blood samples were collected from vaccinated SPF chickens weekly till 4 weeks after inoculation, and ND-HI antibodies were measured in collected sera by HI test. The obtained results pointed that all vaccinated groups of SPF-chicks induced high seroconversion response when ND-HI antibodies were measured in sera of immunized chicks at 3 and 4 WPV. An arithmetic mean of ≥6 log of HI antibodies in serum samples collected 3-4 weeks after vaccination is required for approval.7 Despite, vaccinated SPF chickens were consumed SP in ration exhibited higher immune response than that induced by control treatment group which agreed with Egorova et al,12 that proved the selenium enriched phycocyanin (Se-PC) from food microalgae Spirulina demonstrated significantly increased specific IgG response found the dietary SP increase IgG level in sera of vaccinated chickens.13
Significance between vaccinated SPF chickens groups consumed different SP grams to untreated group exhibited a significance difference (p≤0.005) at 2 WPV of all treated groups except SPF chickens consumed SP with a 0.5 g in kg of ration.
The protective efficacy of inactivated NDV vaccine containing (Ulster 2C strain) were measured through challenging all vaccinated chickens by heterologous local NDV genotype VII,7 stating that an effective ND poultry vaccine should protect at least 90% of vaccinated chickens from death. Another interesting finding was all vaccinated SPF chickens consumed different SP grams confer satisfactory protection which ranged 90-100% against heterologous NDV genotype VII despite, 0.5 g from Spirulina did not revealed significance difference in HI titer to untreated group. These results matched with studies that reported that Spirulina is capable to enhancing non-specific immune responses as well as that the ingestion SP enhanced cell mediated immunity.14,15,16 They revealed the SP produces an immunostimulating effect by enhancing the resistance of humans, mammals, chickens and fish to infections. The vaccinated SPF chickens still untreated couldn't induce the satisfactory protection level which agreed with the same results obtained by Sedeik et al,17 who said that the ND homologous vaccine containing the challenge virus was better for clinical protection than the heterologous vaccine in terms of mortality and body weight loss.
Detection of the oropharyngeal (tracheal) viral shedding post challenge in vaccinated and control groups revealed that, vaccinated SPF chickens groups consumed different SP concentration (0.5, 1, 1.5 and 2g/kg of ration) showed a decrease in the tracheal viral shedding than vaccinated untreated group with a percentage of 55%, 65%, 76%, 87% and 46% of the total collected samples, respectively, but cannot completely prevent viral shedding which agreed with Elshazly,18 who tested the viral shedding in vaccinated chickens after challenge with a NDV genotype VII, and they showed that shedding was significantly reduced in vaccinated groups in comparison with the unvaccinated group but not completely prevented the ND outbreak.
These findings suggest that dietary Spirulina has immune-stimulatory effects on the immune system of SPF chickens. One gram from SP per kg of ration was minimum recommended concentration that able to exhibit optimum immune response, increase protection efficacy against heterologous strains and able to reduce viral shedding.