Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.26 n.1 La Habana ene.-mar. 2009
RESEARCH
Acuabio 1, an enhancer of the immune system in Litopennaeus vannamei shrimps
Acuabio 1, un estimulador del sistema inmune de camarones Litopenaeus vannamei
Ramón Franco1*, Amilcar Arenal1*, Leonardo Martín1, Dayamí Santiesteban1, Jorge Sotolongo1, Rebeca Martínez2, Mario P Estrada2
1Centro de Ingeniería Genética y Biotecnología, CIGB AP 387, Camagüey, Cuba
2Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 entre 158 y 190, Cubanacán, Playa, CP 10400, AP 6162, Ciudad de La Habana, Cuba
ABSTRACT
Acuabio 1 is a mixture of proteins and essential amino acids that exerts a stimulating effect in the development of aquatic organisms. It synchronizes and accelerates fish and shrimp larvae growth on early developmental stages, as well as increases the development and size of these animals. In this work, the stimulating effect of Acuabio 1 on the immune system of Litopennaeus vannamei shrimps was demonstrated by obtaining (5.4 x 106 hemocytes/mL) greater than the control group (4.3 x 106 hemocytes /mL). The hemolymph of treated animals showed higher lectin titers (16) than the control group (8). Animals treated with Acuabio 1 increased their phenoloxidase and peroxidase activities, indicating an enhanced oxidative defense. Furthermore, Acuabio 1 decreased the number of Vibrio spp. and Pseudomonas spp. colony forming units in the culture medium, suggesting a possible prebiotic function. All these properties make Acuabio 1 very attractive to improve the production, survival rate and quality of shrimp larvae.
Keywords: Immune system, Acuabio 1, phenoloxidase, hemocytes, shrimp.
RESUMEN
Acuabio 1 es una mezcla de proteínas y aminoácidos esenciales que ejerce un efecto estimulador en el desarrollo de los organismos acuáticos. Sincroniza y estimula el crecimiento de larvas de peces y camarones. Con su empleo se activa y acelera el mecanismo de crecimiento desde edades muy tempranas, así como el desarrollo y la talla de los animales. En el presente trabajo se evidencia el efecto estimulador del sistema inmune del producto Acuabio 1 en camarones (Litopenaeus vannamei). Se determinó que al tratar los animales con este producto aumentó el número de hemocitos totales (5.4 x 106 hemocitos/mL) con respecto a los del grupo control (4.3 x 106 hemocitos/mL). Se observó que la hemolinfa de los camarones tratados tuvo mayor título de lectinas (16) comparado con el control (8). En los animales tratados con Acuabio 1 se encontró un aumento de las actividades específicas fenoloxidasa y peroxidasa comparadas con las del control, lo que indicó un aumento de la defensa oxidativa en estos animales. También se halló que Acuabio 1 disminuyó el número de unidades formadoras de colonias en el medio para Vibrio spp. y Pseudomonas spp., lo que sugiere una posible función prebiótica. Estas propiedades del producto Acuabio 1 lo hacen muy atractivo para su utilización en el mejoramiento de la producción, supervivencia y calidad de las larvas de camarones.
Palabras clave: sistema inmune, Acuabio 1, fenoloxidasa, hemocitos, shrimp.
INTRODUCTION
The total global production of shrimp culture reached more than 1.5 tonnes in 2002, accounting for 49% of the total shrimp capture. It is estimated an increase (12 to 15%) in this industry every year (1).
Although technological advances in domestication and genetic breeding of some species such as: Penaeus monodon (2) and Litopenaeus vannamei (3) have been made, there is not enough progress, due to the lack of comprehension of genetic and biochemical processes on these species, among other factors.
In order to improve the production process of one species, its genetics, reproduction, nutrition and physiology must be taken into account for the selected organism (4).
The economic relevance of shrimps has motivated its more intensive farming. However, the increased densities of growth have led to epizooties that cause production losses. The use of probiotic bacteria and immunostimulants are among the most used methods to avoid these diseases in shrimps (5).
Acuabio 1 is a precise mixture of proteins and essential amino acids which exerts a relevant nutritional effect at the initial developmental stages in aquatic organisms. Its effects synchronize and stimulate larvae growth, activating and producing the mechanisms of growth from early stages; development and size in fishes, and subsequently increasing resistance to parasites as secondary effect (6).
The properties described make Acuabio 1 very attractive for increasing production, survival and quality of aquatic organisms larvae, the very essential step when culturing them.
Variations have been previously reported in invertebrates in response to several external and artificial stimuli such as: temperature and salinity changes, contaminants, natural or artificially induced infections, among others (7, 8).
In the present work, the effect of Acuabio 1 inparameters of the immune system in Litopennaeus vannamei shrimps is described, including total number of hemocytes, phenoloxidase and peroxidase specific activities and lectin titers, and counts of Vibrio spp. and Pseudomonas spp. in the hepatopancreas.
MATERIALS AND METHODS
Animal culture and treatment
Juvenile, 5.0 ± 0.2 g average weight shrimps were randomly distributed in two groups, with two replicates each. Replicates were seeded in 50 L tanks at 10 animals per aquarium. The control group was treated with 100 mg/L of bovine serum albumin, and the second group was incubated with Acuabio 1 at 100 mg/L. Animals were kept under constant illumination and aeration, at 34 g/L of salinity and 50% of the water was changed daily. Twenty five days later, hemolymph was collected from the ventral sinus at the cephalotorax of all the animals by using 1 mL syringes filled with 387 mM sodium citrate anticoagulant. Samples were transferred to eppendorf tubes and kept in ice (4 ºC) for cell preservation.
Total hemocyte counts
The hemocyte numbers were determined in plasma by cellular counting in a Neubauer hemocytometer by using a phase contrast microscope (Olympus; 40x). The following formula was used for calculation:
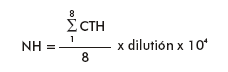
Where:
NH: Number of hemocytes (106 cells/mL)
CTH = hemocytes count in 8 cuadrants
Lectin titers
The used rabbit blood was collected in a solution containing 110 mM D-glucose, 37 mM sodium citrate, 72 mM NaCl and 2.9 mM citric acid, at 1:1 v/v ratio and stored at 4 ºC. The collected erythrocytes was washed twice in PBS 1X (136 mM NaCl, 2.6 mM KCl, 8.0 mM Na2HPO4, 1.5 mM KH2PO4), pH 7.2 Washes were centrifuging at 1500 rpm for 5 min. A 2% v/ v erythrocyte suspension was prepared in PBS 1X, pH 7.2. Serial dilutions were prepared of the interest sam-ples in PBS 1X, pH 7.2, by using a 96-well U bottom plate (Costar®, USA), to a total volume of 100 mL per well. One hundred microliters of the 2% v/ v rabbit erythrocyte suspension were added per well, and the plate was incubated for 1 h at 28 ºC. A negative control of 100 mL of PBS 1X, pH 7.2, was included. The hemagglutination was determined for each sample, and the lectin titer was the highest dilution of the sample where hemagglutination occurred (9).
Antiprotease activity
Twenty microliters of hemolymph were collected per animal and later incubated with a 5 mg/mL trypsin solution for 10 min at 22 ºC. Two hundred microliters of 100 mM phosphate buffer (pH 7.0) and 250 mL of azocasein at 2% w/v were subsequently added, then incubated for 1h at 22 ºC. After that time, 500 mL of 10% w/v trichloroacetic acid were added and incubated for 30 min at 22 ºC. The mixture was centrifuged at 6000 rpm for 5 min and 100 mL of the supernatant was transferred to a 96-well plate filled with 100 mL of 1 M NaOH per well. The reaction was read at 450 nm. Phosphate buffer was used as 100% control instead of hemolymph, and phosphate buffer instead of hemolymph and trypsin. The inhibition percent of trypsin activity was calculated for each sample by comparing the control value. All the samples were analyzed in duplicates (10).
Specific peroxidase activity
It was measured by using pyrogallol (Sigma, USA) as enzyme substrate. Briefly, 64 mL of 100 mM phosphate buffer, pH 6.0, 32 mL of 147 mM H2O2, 64 mL of the 180 mM pyrogallol solution and 420 mL of distilled water were mixed in a 1 cm path length cuvette. 20 mL Twenty microliters of the sample was added to the mixture; then homogenized; later, read its absorbance at 420 nm after 20 s. Distilled water was used as baseline. One peroxidase unit was defined as the amount of enzyme required to catalyze the production of 1 mg of purpurogallin from pyrogallol after 0.20 s at 20 ºC (11). The specific enzyme activity was calculated as the ratio of the enzyme activity over protein concentration. The total protein concentration was previously determined using in 20 mL of hemolymph by the Bradfords method (12).
Phenoloxidase specific activity
It was measured by detecting spectrophotometrically the formation of a chromogen from L-dihydroxyphenylalanine (L-DOPA). Fifteen microliters of hemolymph plasma was placed in a 96-well plate and 50 mL of 7.5 mM L-DOPA were added. The absorbance was measured at 490 after 10 min at 25 ºC. One unit of phenoloxidase was considered as the variation of 0.001 absorbance units per minute (13). The specific enzyme activity was calculated as the ratio of enzyme activity over protein concentration.
Vibrio spp. and Pseudomonas spp. colony number in the hepatopanchreas
One hundred milligrams of hepatopancreas were collected and suspended in 1 mL of sterile PBS 1X. Serial dilutions were carried out in saline (150 mM NaCl) until 10-10, and 100 mL of each dilution were seeded on three plates with agar-thiosulphate-citratebile-sucrose (TCBS; 5 g/L yeast extract, 5 g/L peptone, 10 g/L sodium citrate, 10 g/L Na2S2O3, 8 g/L ox bile, 20 g/L sucrose, 10 g/L NaCl, 1 g/L ferric citrate, 40 mg/L bromothymol blue, 40 mg/L thymol blue, 15 g/L agar; pH 8.6 ± 0.2).
This medium was designed to detect Vibrio spp. and Pseudomonas spp. (14). Once the suspension was absorbed, the plate was incubated at 28 ºC. When growth was observed, the colony forming units (c.f.u.) were estimated by counting the colonies in the dilution showing from 30 to 300 of them.
Statistical analyses
The median of values corresponding to the total hemocyte counts, hemagglutination activity and the number of Vibrio spp and Pseudomonas spp. colonies, were compared by using the Mann-Whitney test. Normality was evaluated for the specific peroxidase and phenoloxidase activities by the Kolmogorov-Smirnov test, followed by a variance analysis by using the F test, also differences between groups were detected. N the case of antiprotease activity, percentage values were transformed by a scale arcsen (X/100)1/2, and the specific enzyme activities were similarly analyzed. Experimental data were statistically evaluated with the aid of the programs Statistical version 6.0 (StatSoft, USA) and GraphPad Prism version 4.00 (GraphPad Software, USA).
RESULTS
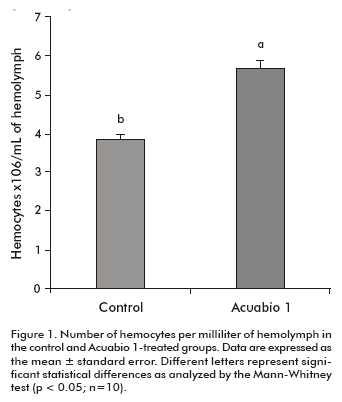
To evaluate the effect of Acuabio 1 on the immune system of L. vannamei, several parameters were measured in the hemolymph to characterize the response against pathogens in peneid shrimps. Animals treated with Acuabio 1 showed a 25% increase in hemocyte counts per milliliter (P < 0.05) compared to untreated animals (Figure 1). This indicates that Acuabio 1 stimulates the proliferation of hemocytes in L. vannamei.
Lectins are among the innate immunity parameters that could be functionally involved in host defense. The agglutinating capacity of hemolymph in shrimps treated with Acuabio 1 was analyzed in rabbit erythrocytes in the presence of lectins. It was observed tha themolymph lectin titers were twice greater than the titers detected in untreated animals (P < 0.05; Figure 2).
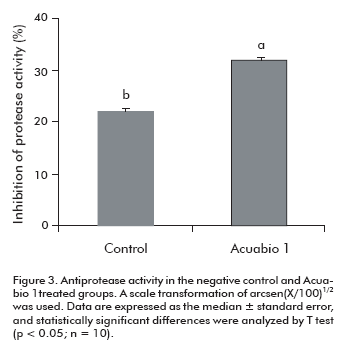
Many bacteria produce proteolytic toxins to digest host tissue proteins as amino acid source. Therefore, the antiprotease activity is another relevant factor of the innate immunity. When L. vannamei shrimps were treated with Acuabio 1, the antiprotease activity in the hemolymph was 50% (P < 0.05) higher than in the control animals.(Figure 3)
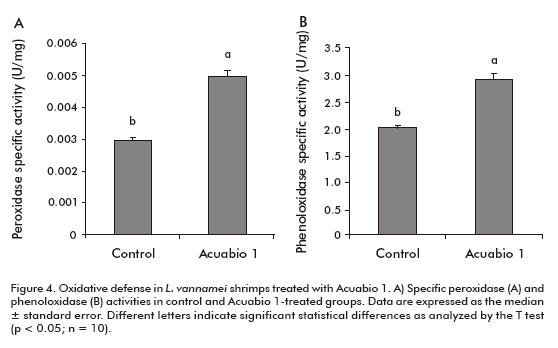
Peroxidase and phenoloxidase activities characterize the oxidative defense in the hemolymph of peneid shrimps. An enhanced specific peroxidase activity (67%; P < 0.05) was detected in the hemolymph of L. vannamei shrimps treated with Acuabio 1, compared to the hemolymph of negative control-treated animals (Figure 4A). The same animals receiving the product increased the specific phenoloxidase activity in 40% (P < 0.05).
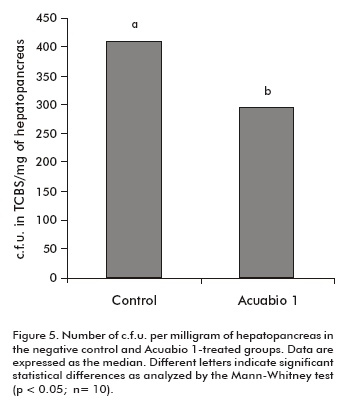
Due to the properties of Acuabio 1, we decided to evaluate its influence on the bacterial flora of the hepatopancreas, specifically Vibrio spp. and Pseudomonas spp. which are potential pathogens in shrimps. Total counts were expressed as c.f.u. per milligram of hepatopancreas, after bacterial growth in TCBS media. It was found that shrimps treated with Acuabio 1 showed a decrease of 40% in the load of Vibrio spp. and Pseudomonas spp. (P < 0.05), compared to animals treated with the negative control (Figure 5).
DISCUSSION
Among invertebrates, crustaceans are a very high economical group due to their easy adaptation to aquaculture. Several crustacean species are affected generally by opportunistic pathogens, being lowered their production. In this work, we showed, for the first time, the influence of Acuabio 1 on the immune system of L. vannamei shrimps. Invertebrates lack not only a humoral immune response based on antibodies but also cellular immune response based on specialized cells as lymphocytes. Therefore, they do not have immune specificity and memory (15). Immunity in crustaceans is mediated by humoral and cellular effectors. Hemocytes remove strange particles in the hemolymph by encapsulation and forming nodular aggregates (16). These cells are additionally involved in wound healing by encapsulation and cellular agglutination, thus initiating the clotting process by releasing the plasma factors required (17). The results obtained evidenced that animals treated with Acuabio 1 significantly increased their total hemocytes in the hemolymph, compared to the control group. Similar results were previously reported for probiotics stimulating the immune system in shrimps (18) , the Bio-Mos® and β-1,3-D-glucan prebiotics (19). A higher total number of hemocytes also increased immune capacity in periods of high metabolic activity (20). Circulating hemocytes counts in the hemolymph has been an indicator of shrimps health because infectious diseases would be modified the composition of hemolymph (21).
Another parameter of crustacean immunity studied was lectin titers. Lectins in decapod crustaceans determine structural properties and specificity as serum lectins in humans. There have being almost completely identified the lectin types of cultured species of shrimp. Lectins in invertebrates are part of the recognition process of strange agents (15, 22), inducing agglutination on pathogenic cells, triggering as opsonins, several cellular events such as: phagocytosis (23). Results showed that shrimps treated with Acuabio 1 developed the highest lectin titers, indicating of an increased agglutinating capacity against pathogens. It was recently identified that the levels of mannosebinging lectins are increased in humans by the administration of immunostimulants (24). Multiple sequence alignments showed that the first carbohydrate recognition domains in the lectin (L v LT) of L. vannamei completely comprise the 23 amino acids of mannose-binding lectins and other types of lectins in fish. The binding of the four residues relevant to form the three carbohydrate recognition domains are completely conserved (25). On the other hand, it has been demonstrated that L. setiferus lectins are involved in the activation of the immune response of granular hemocytes induced by NADPH and other oxidative pathways through a specific receptor (26).
In this study, the antiprotease activity in the hemolymph of animals treated with Acuabio 1 was higher than in the negative control group. There have been described that shrimp plasma contains several protease inhibitors, mainly α1-antiproteinase and α2- microglobulin (27). There were also established that differences in the activity α1-microglobulin in two trout species are related to resistance to Aeromonas salmonicida infections (28). Therefore, an increase in this parameter is beneficial to shrimps health treated with Acuabio 1.
The oxidative defense, represented by the peroxidase and phenoloxydase activities, could be used to measure the health state of the animals (29). Herein, the animals treated with Acuabio 1 showed the highest activities for both oxidative enzymes. The cascade of phenoloxidase activity could derive into the formation of melanin, stimulation of phagocytosis, nodule formation encapsulation and agglutination (30), as well as mediating the synthesis of proteins as antibacterial peptides (31). Plasma peroxidase activity is related to the generation and elimination of reactive oxygen species during phagocytosis (32). An increased oxidative defense in shrimps have been reported after using probiotics (33) and immunostimulants as β-1,3- D-glucan (34, 35).
These results with factors associated to the innate response in shrimps indicated the usefulness of Acuabio 1 as immunostimulant, suggesting that shrimps treated with this product have higher resistance to pathogens under culture conditions. Evidences have been published on the immunostimulating effect of several substances administered to shrimps at different regimes.Nevertheless, data presented were not enough to support their conclusions. In much of these studies, the experimental designs were deficient or statistical limits were absent, being insufficient to validate conclusions (36).
A decreased total number of c.f. u. per milligram of hepatopancreas in TCBS indicated a reduced Vibrio spp. and Pseudomonas spp. flora. Similar results were reported by using the Timsen Ôimmunostimulant (40% alkyldimethylbenzylammonium chloride) in P. monodon shrimps (37). The use of an endogenous immunostimulant, the antilipopolisaccharide factor 3, avoided vibriosis in P. monodon shrimps (38). Our results suggest a prebiotic function of Acuabio1, due to a decrease population of pathogenic bacteria in the hepato-pancreas of shrimps after its administration. Both the prebiotic and the immunostimulating functions make Acuabio 1 in shrimps a highly attractive product for shrimp aquaculture.
REFERENCES
1. Briggs M, Funge-Smith S, Subasinghe R, Phillips M. Introductions and movement of Penaeus vannamei and Penaeus stylirostris in Asia and the Pacific. Serial: RAP Publication (FAO)2004; suppl 2004/10.
2. Macbeth M, Kenway M, Salmon M, Benzie J, Knibb W, Wilson K. Heritability of reproductive traits and genetic correlations with growth in the black tiger prawn Penaeus monodon reared in tanks. Aquaculture 2007;270(1):51-6.
3. Pérez-Rostro CI, Ibarra AI. Heritabilities and genetic correlations of size traits at harvest size in sexually dimorphic Pacific white shrimp (Litopenaeus vannamei) grown in two environments. Aquac Res 2003;34(12):1079-85.
4. Gómez-Chiarri M, Smith GJ, de la Fuente J, Powers DA. Gene transfer in shellfish and algae. In: de la Fuente J, Castro FO. Editors. Gene transfer in aquatic organisms. Austin, Texas: RG Landes Company and Germany: Springer Verlag,1998, p. 07-25.
5. Gullian M, Thompson F, Rodriguez J. Selection of probiotic bacteria and study of their immunostimulatory effect in Penaeus vannamei. Aquaculture 2004;233:1-14.
6. Martínez R, Carpio Y, Gómez Y, Raíces M, Morales A, Herrera F, et al. Acuabio 1 estimula el metabolismo anaerobio y el sistema inmune innato de las larvas de goldfish y tilapia. Biotecnol Apl 2006;23(4):287-94.
7. Vogan CL, Rowley AF. Effects of shell disease syndrome on the haemocytes and humoral defences of the edible crab, Cancer pagurus. Aquaculture 2002;205:237-52.
8. Le Moullac G, Haffner P. Environmental factors affecting immune responses in Crustacea. Aquaculture 2000;191:121-31.
9. Raman T, Arumugam M, Mullainadhan P. Agglutinin-mediated phagocytosisassociated generation of superoxide anion and nitric oxide by the hemocytes of the giant freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol 2008;24:337-45.
10. Bowden TJ, Butler IR, Bricknell IR, Ellis AE. Serum trypsin inhibitory activity in five species of farmed fish. Fish Shellfish Immunol 1997;7:377-85.
11. Wallerstein JS, Alba RT, Hale MG, Levy H. Studies on oxidase activity in potato tubers. Biochim Biophys Act 1947;1:327-34.
12. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteing-dye binding. Anal Biochem 1976;72:248-54.
13. Hernández López J, Gollas Galván T, Vargas Albores FX. Activation of the prophenoloxidase system of the brown shrimp Penaeus californiensis Holmes. Comp Biochem Physiol C 1996;113:61-6.
14. Kobayashi T, Enomoto S, Sakazaki RA, Kuwahara S. A new selective isolation medium for pathogenic vibrios: TCBS Agar. Jap J Bact 19 3;18;391-7.
15. Vázquez L, Pérez A, Millán D, Agundis C, Martin G, Cooper EL, et al. Morphological analysis of haemocytes from the freshwater prawn Macrobrachium rosenbergii. J Morphol 1997;234:147-53.
16. Söderhäll K, Cerenius L. Crustacean Immunity. Annu Rev Fish Dis 1992;2:3-23.
17. Johansson MW, Söderhäll K. Cellular immunity in crustaceans and the proPO system. Parasitol Today 1989;5:171-6.
18. Hai NV, Buller N, Fotedar R. Effects of probiotics (Pseudomonas synxantha and P. aeruginosa) on the growth, survival and immune parameters of juvenile western king prawns (Penaeus latisulcatus Kishinouye, 1896). Aquaculture Res 2009;40:590-602.
19. Hai NV, Fotedar R. Comparison of the effects of the prebiotics (Bio-Mos and [beta]-1,3-D-glucan) and the customised probiotics (Pseudomonas synxantha and P. aeruginosa) on the culture of juvenile western king prawns (Penaeus latisulcatus Kishinouye, 1896). Aquaculture 2009;289:301-306.
20. Truscott R, White KN. The influence of metal and temperature stress on the im-mune system of crabs. Funct Ecol 1990;4:455-61.
21. Perazzolo LM, Gargioni R, Ogliari P, Barracco MA. Evaluation of some hematoimmunological parameters in the shrimp Farfantepenaeus paulensis submitted to environmental and physiological stress. Aquaculture 2002;214:19-33.
22. Mullainadhan P, Renwrantz L. Lectin-dependent recognition of foreign cells by hemocytes of the mussel, Mytilus edulis. Immunobiology 1986;171:263-73.
23. Bayne CJ. Phagocytosis and non-self recognition in invertebrates. Bioscience 1990;40:723-731.
24. Gravholt CH, Leth-Larsen R, Lauridsen AL, Thiel S, Hansen TK, Holmskov U, et al. The effects of GH and hormone replacement therapy on serum concentrations of mannan-binding lectin, surfactant protein D and vitamin D binding protein in Turner syndrome. Eur J Endocrinol 2004;150:355-62.
25. Ma THT, Tiu SHK, He JG, Chan SM. Molecular cloning of a C-type lectin (LvLT) from the shrimp Litopenaeus vannamei: Early gene down-regulation after WSSV infection. Fish Shellfish Immunol 2007;23:430-7.
26. Alpuche J, Rosas C, Vázquez L, Guevara J, Pereyra A, Agundis C, et al. Activation of immunological responses in Litopenaeus setiferus hemocytes by a hemocyanin likelectin. Aquaculture 2009;292:11-5.
27. Armstrong PB, Quigley JP, Rickles FR. The Limulus blood cell secretes 2-macroglobulin when activated. Biol Bull 1990;178:137-43.
28. Freedman SJ. The role of a2-macroglobulin in furunculosis: a comparison of rainbow trout and brook trout. Comp Biochem Physiol B 1991;98:549-53.
29. Hellio C, Nilles AB, Gagnaire B, Renault T, Thomas-Guyon H. Demonstration of a true phenoloxidase activity and activation of a ProPO cascade in Pacific oyster, Crassostrea gigas (Thunberg) in vitro. Fish Shellfish Immunol 2007;22:433-40.
30. Sritunyalucksana K, Söderhäll K. The proPO and clotting system in crustaceans. Aquaculture 2000;191:53-69.
31. Lee SY, Soderhall K. Early events in crustacean innate immunity. Fish Shellfish Immunol 2002;12:421-37.
32. Le Moullac G, Soyez C, Saulnier D, Ansquer D, Christophe J, Levy P. Eject of hypoxic stress on the immune response and the resistance to vibriosis of the shrimp Penaeus stylirostris. Fish Shellfish Immunol 1998;8:621-9.
33. Chiu CH, Guu YK, Liu CH, Pan TM, Cheng W. Immune responses and gene expression in white shrimp Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol 2007;2:364-77.
34. Chang CF, Su MS, Chen HY, Liao IC. Dietary b-1,3-glucan effectively improves immunity and survival of Panaeus monodon challenged with white spot syndrome virus. Fish Shellfish Immunol 2003;15:297-310.
35. Thanardkit P, Khunrae P, Suphantharika M, Verduyn C. Glucan from spent brewers yeast: preparation, analysis and use as a potential immunostimulant in shrimp feed. World J Microbiol Biotechnol 2002;18:527-39.
36. Smith VJ, Brown JH, Hauton C. Immunostimulation in crustaceans: does it really protect against infection? Fish Shellfish Immunol 2003;15:71-90.
37. Sunga HH, Lina SC, Chena WL, Tingb YY, Chao WL. Influence of Timsenk on Vibrio populations of culture pond water and hepatopancreas and on the hemocytic activity of tiger shrimp (Penaeus monodon). Aquaculture 2003;219:123-33.
38. Ponprateep S, Somboonwiwat K, Tassanakajon A. Recombinant antilipopolysaccharide factor isoform 3 and the prevention of vibriosis in the black tiger shrimp, Penaeus monodon. Aquaculture 2009;289:219-24.
* These authors contributed equally to this work
Received in January 2009.
Accepted for publication in March 2009.
Amilcar Arenal. Centro de Ingeniería Genética y Biotecnología, CIGB AP 387, Camagüey, Cuba. E-mail: amilcar.arenal@cigb.edu.cu