Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.31 no.2 La Habana abr.-jun. 2014
RESEARCH
Production of a liquid Bradyrhizobium japonicum inoculant with high impact on the mechanized sowing of soybean in Cuba
Producción de un inoculante líquido de Bradyrhizobium japonicum con alto impacto en la siembra mecanizada de la soya en Cuba
Carmen Menéndez1, Luis E Trujillo1, Ricardo Ramírez1, Dianevys González-Peña2, Davel Espinosa3, Gil A Enriquez1, Lázaro Hernández1
1 Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
2 Instituto Nacional de Ciencias Agrícolas, INCA. Carretera a Tapaste, Km 3½, San José de las Lajas, CP 32700, Mayabeque, Cuba.
3 Unión Agropecuaria Militar Cubasoy. Carretera Venezuela, Km 12, Coralina, Ciego de Ávila, Cuba.
ABSTRACT
Symbiotic nitrogen fixation is critical for high soybean (Glycine max L. Merril) yields with protection to the environment. This paper describes a procedure for massive production of Bradyrhizobium japonicum in an orbital multi-platform shaker and the use of the bacterial culture for seed inoculation in soybean areas under mechanized sowing. The B. japonicum strain was selected based on its highly efficient symbiotic interaction with major soybean varieties cropped in Cuba. Optimization of the growth medium and the use of serial and scaled inoculations allowed maintaining the cultures in continuous logarithmic phase while increasing its volume 100-fold in 7 days. The final culture having average cell viability of 5 × 1010 c.f.u./mL and high exopolysaccharides content does not require additives and can be stably stored for at least 8 months at 4 ºC. Soybean seeds were sprayed with the inoculant at equivalent dose of 150 mL/ha and sowed mechanically in production fields of Ciego de Avila province during the campaigns 2010-2013. The plants showed abundant nodulation and a proper nutritional state during the whole cycle. The bacterium strain was reisolated from root nodules of inoculated plants and its identity was confirmed by phenotype characterization. The high-cell density liquid inoculant rich in polysaccharides produced in this work is compatible with mechanized seed sowing, favors the bacterium survival in soil, and promotes the establishment of an efficient symbiotic interaction with the host plant.
Keywords: Bradyrhizobium japonicum, soybean, Glycine max L., nitrogen fixation, inoculant.
RESUMEN
La fijación simbiótica del nitrógeno es un proceso clave en la obtención de altos rendimientos en el cultivo de la soya (Glycine max L. Merril) con protección del medio ambiente. Se describe una metodología para la producción masiva de una cepa de Bradyrhizobium japonicum de alta eficiencia simbiótica, en una zaranda orbital de plataformas múltiples, y el empleo del cultivo bacteriano como bioinoculante para variedades de soya bajo condiciones de siembra mecanizada en Cuba. La optimización del medio de crecimiento y el establecimiento de un sistema de inoculaciones seriadas y escaladas permitieron mantener el cultivo en continua fase logarítmica e incrementar su volumen 100 veces en 7 días. El cultivo final con viabilidad promedio de 5 × 1010 u.f.c./mL y alto contenido de exopolisacáridos no requiere aditivos y se mantiene estable en conservación a 4 ºC durante al menos 8 meses. La aplicación del inoculante a las semillas fue a dosis equivalente de 150 mL/ha durante las campañas de 2010-2013 en la empresa Cubasoy, de la provincia Ciego de Ávila. Las plantas de campo inoculadas mostraron nodulación abundante en la zona del cuello de la raíz y mantuvieron un estado nutricional adecuado durante todo el ciclo vegetal. La bacteria reaislada de nódulos de raíces en plantaciones al azar mostró el mismo fenotipo de la cepa inoculada, lo que confirma la efectividad de la inoculación. El inoculante líquido obtenido es técnicamente compatible con el empleo de las máquinas sembradoras, favorece la supervivencia de la bacteria en el suelo y su simbiosis eficiente con la planta hospedera.
Palabras clave: Bradyrhizobium japonicum, soya, Glycine max L., fijación de nitrógeno, inoculante.
INTRODUCTION
Soybean (Glycine max L. Merril) is a high nutritional value legume, its beans containing up to 30 % of proteins. They provide all the essential amino acids but not methionine. This deficiency can be compensated by diet combinations with cereals as recommended in classical dietary procedures [1]. Soybean is also used as protein supplement for animal feed. In the last years, the world soybean production surpassed 250 million tons, led by US, Brazil, Argentina, China, India, Paraguay and Canada [2]. In Cuba, soybean production recently arose as an economical policy priority, in order to substitute grain import, with a remarkable raise in the number of areas destined to soybean crops and the introduction of mechanized sowing.
It has been estimated that the soybean plant requires up to 80 kg of assimilable nitrogen to produce a ton of grain, accounting for 240 kg/ha in average. Nitrate or ammonium becomes available in soil by organic nitrogen mineralization, chemical fertilization, and biological nitrogen fixation. The latter process is essential for nitrogen incorporation to the biosphere. The conversion of atmospheric nitrogen into ammonium conducted by microorganisms bearing the enzyme nitrogenase is an intrinsic non-contaminating process that prevents soil impoverishment [3]. At the same time, high concentrations of nitrate or ammonium in soil inhibit the nitrogen biological fixation.
The specific interaction between rhizobia and legume plants results in the most efficient form of biological nitrogen fixation, known as symbiotic nitrogen fixation, accounting for 60-80 % of total fixed nitrogen in nature. The rhizobium-host plant interaction leads to the formation of nodules, specialized structures generally found in roots, providing an ideal microenvironment to reduce gaseous nitrogen to ammonium. In this symbiotic interaction, the plant provides the carbon source for bacterial growth in exchange of the fixed nitrogen.
The soil bacterium Bradyrhizobium japonicum establishes symbiotic nitrogen fixation specifically with soybean [4]. This bacillus-shaped Gram-negative species produces abundant exopolysaccharides which display specific functions as carbon source and protective barriers at the initial colonization steps during the bacterium-host plant interactions, increasing bacterial survival in the soil under adverse conditions. Two other Bradyrhizobium species, B. elkanii and B. liaoningense are capable to nodulate soybean [5]. B. japonicum shows a slow growth in culture and has been extensively used to produce liquid and solid bioinoculants for application in seeds before sowing [6]. Solid inoculants are composed of irradiated peat or other support that contains the culture broth and, once applied to seeds, favors bacterial survival in soil. That is why seeds pelleting with solid products is the inoculation method of choice for manual sowing. However, the use solid inoculants tend to cause mechanical failures in sowing machines. Instead, seeds are recommended to be treated with liquid inoculants that guarantee high bacteria levels in soil.
For that purpose, we describe a method for large scale production of high cell-density cultures of B. japonicum under optimized shaking conditions. The liquid inoculant enriched of natural exopolysaccharides and free of microbial contaminants was stable at 4 ºC for at least 8 months. It was effectively used for extensive mechanized sowing of soybean in four production campaigns from 2010 to 2013, in production fields of Ciego de Ávila province, Cuba.
Materials and methods
Bradyrhizobium strains and soybean cultivars
Bradyrhizobium japonicum strains Semia 5079 and Semia 5080 and B. elkanii strain ICA 8001 [5] were used for comparisons on the efficiency of symbiotic interaction with soybean cultivars IncaSoy-36 [7], Conquista [8] and CubaSoy-23 [9], under greenhouse conditions. The strain Semia 5080 was used for massive production of the inoculant.
Culture media
Five culture medium variants were generated from the commercial broth YM (10 g/L mannitol, 1 g/L yeast extract, 0.5 g/L K2HPO4, 0.2 g/L MgSO4 · 7 H2O, 0.1 g/L NaCl) (Table 1) [10]. The starting pH was adjusted to 6.8 and kept unbuffered during bacterial growth. The medium generating the highest biomass yield was named YMG and further used for massive inoculant production.
Bacteroid isolation from nodules
Plant roots inoculated and grown under greenhouse or field conditions were washed with distilled water and air-dried at room temperature. Bacteroids were isolated from active nodules (inner red pigmentation) at the root crown from flowering plants. Nodules were manually collected, submerged in 70 % ethanol for 5 min for superficial decontamination and transversally sliced with a sterile scalpel under a vertical flow air cabinet. Bacteroids were collected from the central area of the nodules with a sterile loop and seeded in Petri dishes containing solid YM medium, supplemented or not with 100 mg/L carbenicillin and the colorant Congo red (0.25 %, w/v). Whitish mucoid colonies characteristics of Bradyrhizobium appeared after culturing for 10 days at 28 ºC.
Selection of Bradyrhizobium strain and establishing the primary cell bank
Experiments evaluating the efficiency of the symbiotic interaction between three Bradyrhizobium strains and three soybean cultivars were run on zeolite substrate without nitrogen fertilization under greenhouse conditions. One week after seed germination, plantlets were inoculated with 1 mL of bacterial culture diluted at 2 ×106 viable cells/pot. Plants were collected at flowering and symbiosis efficiency indicators were quantified (Table 2).
For preparation of the primary cell bank (PCB), B. japonicum strain Semia 5080 was recovered from a root nodule of a previously inoculated soybean plant cv. CubaSoy-23 grown until flowering stage under greenhouse conditions. A single colony was obtained from a bacteroid isolated from a root nodule of a CubaSoy-23 soybean flowering plant previously inoculated with a pure bacterial culture and grown under greenhouse conditions. The colony was inoculated to a preculture containing 5 mL of liquid YM medium, and further incubated at 28 ºC for 3 d under roller shaking. This culture was used as inoculums for 300-mL YM medium cultures and incubated at 28 ºC under shaking until reaching logarithmic growth The homogeneously pure culture, as evidenced by Gram-staining, was centrifuged and cells collected, washed with sterile YMG medium and further resuspended in cold YMG medium supplemented with 30 % glycerol (v/v), dispersed in cryopreservation vials on ice and stored at –70 ºC until use. The PCB showed a cell viability of 107 c.f.u./mL and was free of microbial contaminants, receiving the approval certification by the Quality Control Unit of the Center for Genetic Engineering and Biotechnology (CIGB), in Habana, Cuba.
Inoculant production, quality control and stability under preservation
Single colonies were obtained from 1/106 and 1/107 dilutions from a PCB aliquot of the B. japonicum strain Semia 5080, plated on YMG medium supplemented with 100 mg/L carbenicillin and the colorant Congo red (0.25 %, w/v). After 10-d incubation at 28 ºC, the colonies were not red stained and showed the mucoid phenotype indicative of exopolysaccharides production. The primary inoculums were prepared in 5 mL YM cultures from single bacterial colonies, grown at 28 ºC for 3 d under roller shaking. These cultures were subsequently used to prepare the secondary inoculums, by inoculating 15 mL to Erlenmeyer flasks containing 150 mL of YMG medium, and cultures were further incubated at 28 ºC for 3 d in an orbital shaker. The final large scale culture was established by inoculating 300 mL of the secondary inoculums into Erlenmeyers containg 3 L of YMG medium, which were incubated at 28 ºC for 4 d and under 150 rpm agitation. Each final culture was tested for microbial purity by Gram-staining and optical microscopy. The final homogeneous cultures showing the characteristic appearance of homogeneous B. japonicum Gram-negative bacilli were pooled in 5-L batches, stored in plastic barrels and preserved at 4 ºC until commercialization. Batches were tested for purity by the streak plate method in plates containing culture medium stained with Congo red and devoid of antibiotics. Viability was determined by the serial dilution method [10] and colonies were counted in Petri dishes filled with YM medium supplemented with 100 mg/L carbenicillin (not inhibiting B. japonicum Semia 5080) and Congo red staining (0.25 %, w/v). Batches of the liquid inoculant were efficacy tested by spraying it to seeds at soybean field doses, which were subsequently sowed in zeolite-containing pots. Plants were grown for 30 days under greenhouse conditions without nitrogen fertilization, and nodulation was corroborated in roots at that time.
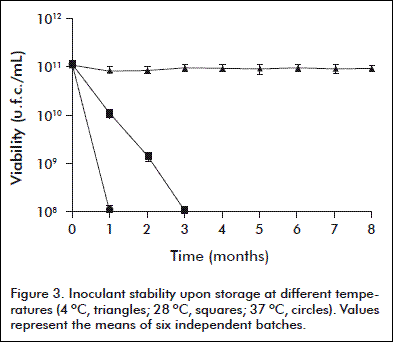
The inoculant stability was assessed during storage at 4, 28 y 37 ºC for 8 months. Six batches were evaluated for viability and purity for each temperature, in 1 mL monthly-collected samples.
Seed inoculation under field conditions by mechanized sowing
Certified seeds of the soybean cultivars tested were sprayed with the inoculant at the equivalent dose of 150 mL/ha, before mechanized sowing in ferralitic soils at the areas of the Cubasoy Enterprise, in Ciego de Ávila province, in Cuba, in the production campaigns 2010-2013. Prior to seed sowing, the soil was fertilized with the macronutrients nitrogen, phosphorous and potassium at respective doses of 50, 60 and 45 kg/ha. No urea was applied during plant cultivation. The effectiveness of the inoculation was determined by evaluating nodulation (root nodule distribution, number, size and internal color) and the absence of symptoms revealing plant nutritional deficiency.
RESULTS AND DISCUSSION
Selection of a B. japonicum strain of high symbiotic efficiency with major soybean (Glycine max L.) cultivars in Cuba
The symbiotic efficiency of three commercial strains of Bradyrhizobium was tested in interaction with three soybean cultivars (IncaSoy-36, Conquista, CubaSoy-23) grown in zeolite substrate without nitrogen fertilization under greenhouse conditions. All the treatments induced abundant nodulation regardless the differences in plant foliar mass (Table 2). The inoculation of strain ICA 8001 B. elkanii induced the highest number of nodules for the three soybean cultivars, with no significant differences in the average fresh weight of the nodules. The highest foliar mass was detected for strain Semia 5080, this strain being selected for bioinoculant production. In all the cases, nodules were widely distributed at the root crown area (Figures 1A and 1B) and showed the internal red color (Figure 1C) characteristic of the protein leghemoglobin, whose presence indicates the efficient occurrence of the nitrogen fixation process. Plants inoculated kept intense green during the experiment.
Establishing a cost-affordable growth medium for culturing B. japonicum at high cellular densities
The YM commercial broth is widely used to culture Rhizobium and Bradyrhizobium species at laboratory scale. Nevertheless, the use of mannitol as the sole carbon source makes extremely expensive the large scale production of inoculants. The growth of the B. japonicum Semia 5080 strain in YM medium was compared to that in other five culture media (Table 3). The replacement of mannitol by glycerol as carbon source in medium 1 notably reduced polysaccharide production in culture. Exopolysaccharides are primary components of the biofilm that protects bacteria from desiccation in soil, functions as reserve energy source, and mediates rhyzobial interaction with the host plant [11]. An efficient exopolysaccharide production is also important to increase the culture viscosity which favors the adhesion of B. japonicum to seeds upon inoculation [12, 13]. The combination of 2 g/L mannitol with 1.2 % glycerol in culture media 2 to 5 reduced their costs without compromising exopolysaccharide production, as compared to the YM control medium. The further increase of the yeast extract content in media 4 and 5, whose consumption
generates ammonia, allowed the bacterium growth to occur at optimal neutral pH values [10]. The ultimate addition of nitrogen salts in medium 5 further contributed to increase the cellular density up to 17.2 g of wet biomass per liter of culture, achieving viable counts above 5 × 109 c.f.u./mL. These results and the low cost of raw materials (0.67 USD/L) led us to select medium five, named YMG, for the inoculant production.
Batch production of high cell density cultures of B. japonicum in an orbital multi-platform shaker
Opposed to species of the genus Rhizobium species, B. japonicum displays a slow growth in culture [4] that makes challenging its large scale production at batch scale. A system was established comprising two 1/10 serial dilutions, which allowed to maintain bacterial growth steadily at logarithmic phase in YMG medium and to achieve a high average viability (5 ×1010 c.f.u./mL) in the final culture. The entire culturing process from colony inoculation to the harvest of the final bacterial culture lasted 10 days. Nevertheless, the batch culture in the shaker took only 7 days the culture volume was increased 100-fold in comparison to initial precultures. We overlapped in time the growth phases of the secondary inoculums and the final culture in the multiplatform orbital shaker allowing the production of two inoculant batches in a week.
The quality standards established for the inoculants commercialization were the absence of microbial contaminants and the achievement of cellular densities of at least 5 × 109 c.f.u./mL. This cell viability allows to administer 6 × 106 viable bacteria per seed, according to the field application dose of 150 mL/ha. Homogeneous Gram-negative bacilli populations were observed under the microscope consistent with Bradyrhizobium morphology in all the growth phases in batch cultures. The lack of microbial contaminants was then confirmed by plating inoculants batch samples on solid YM medium supplemented with Congo red and devoid of antibiotics. In that medium, B. japonicum forms whitish mucous colonies that do not absorb the colorant. The use of the fast Gram-staining method followed by microscopic observations of secondary inoculums allowed minimizing the rejection rate of final cultures to less than 2 % due to bacterial contamination. The results of efficacy testing of three batches of the inoculant evaluated under greenhouse conditions are shown in figure 2.
Inoculated plants developed intense green vigorous foliage during the experiment, compared to the untreated controls (Figure 2A), as well as abundant nodules in the root crown (Figure 2B).
Preservation of the liquid inoculant
The stability of the inoculant during storage at 4, 28 and 37 ºC for 8 months is shown in figure 3. The refrigerated preservation did not decrease significantly the viability of the inoculant. Otherwise, viable counts were lower than the specified standard limit (5 × 109 c.f.u./mL) when stored at 28 and 37 º C for one and two months, respectively.
Efficiency of inoculant application in soybean fields under mechanized sowing conditions
Soybean is mechanically sowed in the Ciego de Ávila province, in Cuba, which makes impossible the use of peat-based traditional solid inoculants. For this reason, liquid inoculant batches produced as described in this work were used for seeds treatment at dose of 150 mL/ha in the production campaigns 2010-2013, resulting in a yearly average consumption of 800, 570, 900 and 1030 L. Soybean plants at flowering phase were sampled each campaign and evidenced abundant nodules with nitrogen-fixing activity (internal red color), mainly located at the root crown (Figure 4A). Plantations showed an excellent nutritional state with vigorous and intense green foliage. Phenotype characterization studies of the bacterium isolated from nodules of randomly selected plants corroborated the identity of the inoculated Bradyrhizobium strain. The identity criteria used were: resistance to 100 mg/L carbenicillin and rifampicin 100 mg/L, production of whitish mucous colonies on solid YM medium supplemented with Congo red (Figure 4B) and no acidification of YM medium supplemented with the pH indicator bromotimol blue (Figure 4C).
The high cellular density liquid inoculants rich in polysaccharides was technically compatible with the use of sowing machines, allowed the survival of bacteria in soil and promoted the establishment of an efficient symbiotic interaction with three major soybean varieties cropped in Cuba. Under the non-inhibitory concentrations of chemical nitrogen in soil, the symbiotic process provided enough amounts of fixed nitrogen for the proper plant development, contributing to the photosynthesis and aiding to reach high yields of grains. The foliar residues of soybean crops returned part of the fixed nitrogen to the soil, with collateral benefits for the gramineous rotation crops, mainly maize.
In summary, a batch production system was established to obtain a liquid inoculant of B. japonicum, of high cellular density and rich in exopolysaccharides. The inoculant was stable for at least 8 months under refrigerated storage at 4 ºC. The technological scale up is simple and requires neither large facilities nor expensive equipment. Seed inoculation in the field was compatible with the use of sowing machines, promoted abundant nodulation in plants and they developed and a proper nutritional state during the whole cultivation cycle. The substitution of chemical nitrogen fertilizers by the inoculant reduces the costs of the national soybean production and also contributes to protect the environment.
REFERENCES
1. Producción de determinados productos básicos agrícolas, Grupo I (2004). In: FAO. Anuario estadístico de la FAO. 2005-2006. Roma: FAO; 2004. p. 79-82.
2. Clive J. ISAAA Brief 42. Global Status of Commercialized Biotech/GM Crops: 2010. New York: International Service for de Acquisition of Agri-Biotech Applications (ISAAA); 2010.
3. Singh MS. Effect of Bradyrhizobium inoculation on growth, nodulation and yield attributes of soybean - A Review. Agric Rev. 2005;26(4):305-8.
4. Appelbaum, E. The Rhizobium/Bradyrhizobium-legume symbiosis. In: Gresshoff PM, editor. The molecular biology of symbiotic nitrogen fixation. Boca Raton: CRC Press; 1989. p. 131-58
5. Hollis AB, Kloos WE, Elkan GE. DNA:DNA hybridization studies of Rhizobium japonicum and related Rhizobiacea. J Gen Microbiol. 1981;123(2):215-22.
6. Alberton O, Kaschuk G, Hungria M. Sampling effects on the assessment of genetic diversity of rhizobia associated with soybean and common bean. Soil Biol Biochem. 2006;38(6):1298-307.
7. Soto N., Ferreira A, Delgado C, Enríquez GA,. Regeneración in vitro de plantas de soya de la variedad cubana IncaSoy-36. Biotecnol Apl. 2013;30(1):29-33.
8. Romero A, Peña L, González M, González M. Evaluación de cultivares de soya (Glycine max) en las condiciones edafoclimáticas del municipio Majibacoa, Las Tunas. Innov Tecnol. 2013;19:1-9.
9. Díaz MF, Padilla C, Torres V, González A, Curbelo F. Caracterización bromofológica de variedades de soya (Glycine max) en producción de forrajes, forrajes integrales y granos de siembras de verano. Rev Cubana Cienc Agríc. 2003;37(3):311-7.
10. Somasegaran P, Hoben HJ. Handbook for rhizobia. Methods in legume-Rhizobium technology. New York: Springer-Verlag New York, Inc.; 1994.
11. Subba Rao NS. Soil microorganisms and plant growth. New Delhi: Oxford and IBH Publishing Co.; 1977.
12. Louch HA, Miller KJ. Synthesis of a low-molecular-weight form of exopolysaccharide by Bradyrhizobium japonicum USDA 110. Appl Environ Microbiol. 2001;67(2):1011-4.
13. Maurice S, Beauclair P, Giraud J, Sommer G, Hartmann A. Survival and change in physiological state of Bradyrhizobium japonicum in soybean (Glycine max L. Merril) liquid inoculants after long-term storage. World J Microbiol Biotech. 2001;17(6):635-43.
Received in December, 2013.
Accepted in March, 2014.
Carmen Menéndez-Rodríguez. Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba. E-mail: carmen.menendez@cigb.edu.cu.