Introduction
Epidermal growth factor (EGF), although one of many growth factors, is the prototype of the EGF ligand family, which comprises several proteins involved in cell proliferation, migration and survival. The number of studies on its biology and implications remains relatively low, in contrast to the scientific output related to its receptor and other members of this protein family.1,2,3,4
It is a small protein, with only 53 amino acids and an approximate weight of 6kDa. Despite its size, it is very stable, thanks to the presence of 3 disulfide bridges (Cys6-Cys20, Cys14-Cys31, Cys33-Cys42) throughout its structure, which contributes to its thermodynamic stability. It was initially described in samples from murine submaxillary glands and human urine, although the predominance of platelet origin in relation to its concentrations in blood is currently a widely accepted canonical position.5,6,7
Its membrane precursor is a transmembrane protein weighing about 170 kDa with about 1207 amino acids, called prepro-EGF. The extracellular domain is large, having 8 regions of sequence partially homologous to mature EGF, with approximately 40 amino acids, including 6 cysteines (Cys) spaced as in EGF. Mature EGF constitutes only 5 % of the precursor molecule and is located in the extracellular domain close to the cell membrane. It has been proposed that prepro-EGF may function as a membrane receptor for an unidentified ligand.8
In the therapeutic context, modulation of serum levels has demonstrated important effects. Cuban biotechnology is a pioneer in the development of therapeutic strategies based on EGF. New studies also show promising results in neurological pathologies of ischaemic origin and the pulmonary sequelae of COVID-19.9,10
Current methodologies on enzyme-linked immunosorbent assay platforms are the most widespread for the measurement of EGF values in a biological fluid. A correct assessment of the biological implications of its measurement is essential for a sufficient understanding of the value obtained in relation to an interpretation in tune with its biological function. As an initial approach, the aim of this study was defined to describe the effect of the modification of the sample collection procedure for EGF determination and the possible biological implications of its results.
Method
In the casuistry, a cross-sectional observational study was carried out, recruiting 37 apparently healthy working age individuals. Diagnoses were performed by experienced, certified professionals. Compliance with the medical check-up plan, regular analyses (haemochemistry) and a physical examination, which were essential in certifying their status as apparently healthy individuals, were certified.
The clinical and analytical data were collected by the professional teams in care of the patients in the corresponding medical institutions. The information used was collected from medical records, including age, sex and skin color. It was verified that the acquisition of the analytical parameters was carried out in strict compliance with the standardized procedures of the clinical laboratory of the institutions and in accordance with the regulations in force at CECMED. Only data that were of interest to the study are shown.
Serum EGF detection was performed using the commercial kit UMELISA-EGF from the Cuban Immunoassay Centre. In all cases, 5 ml of blood was collected by puncture of the cephalic vein in the flexure of the arm using disposable syringes of 10 mL capacity, with 21 G hypodermic needles, deposited in a dry test tube, obtaining the serum by the clot retraction technique for 4 hours and centrifugation (according to the manufacturer's recommendations at 1500 rpm for 10 minutes at 240 C). The serum obtained was dispensed by eppendorf micropipettes into 1.5 mL eppendorf vials, after which they were stored frozen at -200 C, until processing at the SUMA laboratory, certified by CECMED, at the Juan Bruno Zayas Hospital.
An experimental variation in the handling of samples for EGF determination was used to estimate the possible impact of platelet lysis on the absolute value of serum EGF concentrations. The experimental procedure followed in this case involved centrifuging at 4o C and applying a successive sequence of freezing/thawing of the sample, in order to accelerate platelet lysis; applying the methodological recommendations implemented by the SAMERSAC service of the Renato Guitar Rosell Blood Bank, a leader in the collection and use of platelet factors.11
Results were expressed in picograms per millilitre (pg*mL-1).
A digital database was used to record the variables, created using the technical facilities of the Excel software of the Microsoft office 2010 platform (Microsoft, USA), on a Hewlett-Packard laptop computer. Data processing was carried out on the same technological platform. In the statistical analysis, measures of central tendency (arithmetic mean), dispersion (standard deviation and confidence interval) and Pearson's correlation coefficient were used as summary parameters. Q-Q and Jarque-Bera (JB) normality tests were performed; in addition, the statistical significance of observable differences between groups was explored with the chi-square test (categorical variables) or Welch's t-test (continuous variables), and the effect size was estimated using Hedge's g-formula. To determine whether or not there is a statistically significant difference between the means of three or more groups, an analysis of variance test (ANOVA) was performed.
The results of the study are part of the project: Role of Epidermal Growth Factor in the aetiopathogenesis and pathophysiology of inflammatory pneumopathy in the context of the SARSCoV-2 pandemic (PS24SC1223). The study was designed and conducted according to the general principles set out in the documents adopted by the international community in relation to biomedical research in humans, contained in the Declaration of Helsinki (update of the World Medical Assembly held in Brazil, 2013), with the state regulations in force according to the requirements of the national regulatory authority (Regulation 165/2000 of the CECMED), as well as the Good Clinical Practice Guidelines of the International Conference on Harmonisation (ICH E6). The research was approved by the Research Ethics Committee of the Saturnino Lora Hospital, and the corresponding certification by the Regional Ethics Committee of the southeast region of Cuba. Prior to the inclusion of each subject in the study, informed consent was requested and obtained. No third party funding was received for the conduct of this research, nor did the subjects involved in the study receive any payment.
Results
The mean age of the sample studied was 45.89± 3.35 years, ranging from 27 to 62 years. The female sex predominated in a 1.8:1 ratio with respect to the male sex. Subjects with mixed skin color predominated. As shown in Table 1, 13.7 % of the subjects had a history of Covid-19 in the 6 months prior to sampling.
Table 1 General characteristics of the study sample.
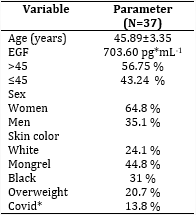
*History of Covid-19 in the 6 months prior to sampling.
Source: Database.
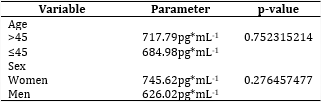
There were no significant differences in EGF values, according to the standard method, between sexes or age groups, as shown in table 2.
The mean EFG value, applying the modified procedure was 2209.47 ±125.73 pg*mL-1, for female subjects was 2218.49±148.80pg*mL-1 compared to 2174.88±313.28 pg*mL-1 for male subjects, without being different (p=0.7648).
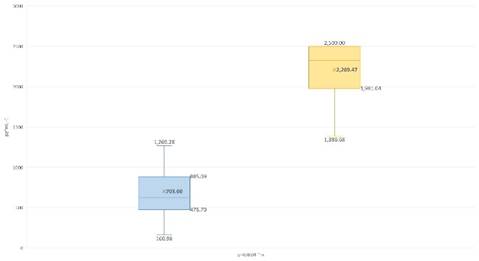
The application of the experimental procedure showed significant and noticeable differences between the EGF values in relation to the sample processing (g=17.7; p=0.0001-23), as shown in fig 1. No linear correlation was observed between the values (r=0.1305; p=0.6843).
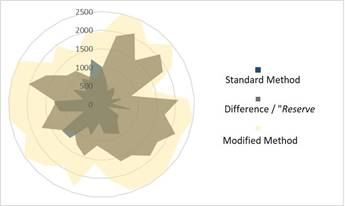
The difference between the value obtained with the modification and the standard procedure behaves as an independent data set, intermediate between the two sets of starting data. The average value was 1498.61±160.68 pg*mL-1. The three data sets did not reflect equality (p=0.0003-23). With respect to the typical EGF value, the differences were large (g=7.976), as well as with respect to the alternative EGF values (g=7.0291). This "reserve" value behaves similar to a transition value, as shown in fig 2.
Discussion
In this study, there were significant and marked differences between the EGF values obtained by the standard method in the context of coagulation and the alternative procedure where platelets were pre-isolated. No linear correlation between EGF values was observed. The difference between the value obtained with the modification and the standard procedure behaves as an independent data set, intermediate between the two starting data sets. With an average value with a large difference in effect in relation to the initial data. This value, which is assumed to be a kind of "reserve" of EGF contained in the platelets, behaved similarly to a transition value.
These results can be interpreted by considering that, from its precursor, EGF is released from the biological membrane by enzymatic cleavage. However, considering that the main source of EGF transcripts is located in the kidney, weighing the organ-specific contribution to blood EGF values is of particular value.12
In this direction it is important to note that it is currently assumed that EGF obtained from blood, in the context of coagulation, is mainly from platelets, but many factors must be considered:
levels of free and membrane-associated EGFR,13
levels of proteolytic Ez released from neutrophilic nuclear polymorphs and other cells with potent lysosomal machinery,14
the direct contribution of platelets, platelet membrane and platelet granules,15
structural and functional integrity of the kidneys and glomerular membrane.16
In the first case, these variable EGFR levels may be associated with a capture phenomenon, given the high EGF-EGFR affinity; although very low levels of free EGFR are generally reported, and EGFR expression at the blood cell membrane level is irrelevant.
In the second case, the possible imprinting is more plausible, given the specialization of many of the nucleated cells of the immune system present in the blood. However, the freeze/thaw process could mitigate this presumable factor, as it may involve the change in activity of associated proteolytic enzymes built into the lymphocytes, which would reduce EGF clearance, favoring a positive balance.
The third case is the most curious to analyze. González Perez I17 has reported that by varying the waiting time for clot retention from 1 hour to 4 hours, a marked increase in EGF values detected in the serum sample can be observed; that is similar to the reported by Savage.18 However, their analysis of the results, applied to the niche of lung cancer patients, biases a view that focuses the result on a direct effect of the tumor on circulating EGF when, in fact, the temporal variation, if it were exclusively tumor-dependent, in a sample outside the organism should decrease. A valid question to ask is: In the context of cancer, as part of the body's reparative response, is there a medullary release of platelets with increased EGF expression? Does this measure of response and its variability in itself reflect disease progression, or to some degree serve as a prognostic indicator? Finally, despite the existence of many other factors, the role of the kidneys and the integrity of the glomerular membrane are, in the author's opinion, important factors in the approach to the phenomenon. Although there is experimental and clinical evidence of the relevance of EGF determinations in urine,19 and the reduction of the presence of this molecule as expressions of glomerular damage,20 the correlation with blood concentrations is still not sufficiently explored, nor to what extent one depends on the other. With this theoretical approach, it is plausible to assume the existence of a platelet reserve, which may be a reflection of an organic response, depending on the existence of a cause that underlies a medullary response for increased EGF release in response to tissue damage. The monitoring of this value of EGF, presumably associated largely with the inner side of platelets, may be variable in certain pathological contexts, especially depending on how strongly damage per se stimulates the body's reparative response. In the particular case of the oncological context this may be expressed differentially, as high values (associated with autocrine production) or low values (dependent on increased consumption), whereby each particular disease may express differential serum patterns, and it would be a mistake to rush to classify an increase as exclusively negative. Conclusively, these results contribute to a novel approach to interpret the biological significance of EGF values, which in particular pathological contexts may be of greater relevance than those obtainable with the standard procedure; distinguishing and measuring platelet contribution is essential to reflect possible differences and interpret them. They are also a basis for the exposition of further scientific questions; further studies are necessary, but experimental evidence, as a result of this first approximation, indicates the existence of higher EGF values after platelet isolation than those observed in serum samples suggesting, a posteriori, a consumption effect in the course of platelet lysis under in vitro conditions; the existence of an intraplatelet EGF pool is plausible. The author collaborates scientifically with the Neuroprotection Group of the Centre for Genetic Engineering and Biotechnology, as well as with the Directorate of Clinical Research of the Centre for Molecular Immunology.