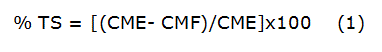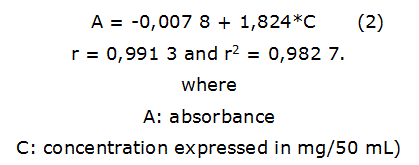Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Química
versión On-line ISSN 2224-5421
Rev Cub Quim vol.29 no.3 Santiago de Cuba set.-dic. 2017
ARTICULOS
Chemical composition and larvicidal activity of cashew nutshell ethanolic extract against mosquito larvae
Composición química y actividad larvicida de un extracto de marañón frente a larvas de mosquitos
Dr. C. Jesús Rafael Rodriguez AmadoI, Dr. C. Raimundo Nonato Picanço SoutoII, MSc. Miriam Santos MagalhãesII, Dr. C. Julio César Escalona ArranzIII, Dr. C. Jose Carlos Tavares CarvalhoI
ILaboratório de Pesquisa em Fármacos. Faculdade de Ciências da Saúde. Universidade Federal do Amapá, Macapá, AP. Brasil, jiribilla2009@gmail.com
IILaboratório de Arthropoda. Faculdade de Biologia. Universidade Federal do Amapá, Macapá, AP. Brazil
IIIDepartament of Pharmacy, University of Oriente, Santiago de Cuba, Cuba, jcea@uo.edu.cu
ABSTRACT
The goal of this work is to evaluate the chemical composition and the larvicidal activity of an extract from the nutshell (Anacardium occidentale L.) against Aedes aegypti and Culex quinquefasciatus larvae. A Hydro-ethanolic extract was prepared by maceration using 1 g of cashew nutshell in 3 mL of ethanol 70 %. The larvicidal effect and the acetylcholinesterase inhibitory activity of the extract were evaluated. The extract content includes phenolic compounds, coumarins, quinones, anthocyanidins, triterpenes, steroids, flavonoids, saponins, lipid compounds, aliphatic acids, and terpenoids. The larvicidal activity of the extract (LC50) for A.aegypti was 35,81 ppm and 40,21 ppm for A. aegypti (Macapá and Rockefeller strains), and 21,47 ppm for C.quinquefasciatus. The IC50 for the AChE inhibitory test was 43,27 ± 2,22 ppm. The evaluated extract presents a good AChE inhibitory activity and a potent larvicidal activity. The larvicidal effect could be associated with the AChE inhibitory activity of the extract.
Keywords: larvicidal activity, nutshell ethanolic extract, mosquitoes.
RESUMEN
El objetivo de este trabajo es evaluar la composición química, así como la actividad larvicida contra mosquitos Aedes Aegypti y Culex quinquefasciatus de un extracto de la cáscara de nuez de Anacardium Occidentale L. o marañón. Con esta intención, un extracto hidro-etanólico es preparado por maceración y en proporción de 1 g de corteza seca en 3 ml de etanol al 70 %. Al extracto se le determinó la presencia de compuestos fenólicos, cumarinas, quinonas, antocianidinas, triterpenos, esteroides, flavonoides y saponinas. La actividad larvicida mostró una concentración letal media (LC50) de 35,81 ppm y 40,21 ppm para larvas de A. aegypti (cepas Macapá y Rockefeller respectivamente) y 21,47 ppm para C. quinquefasciatus. La LC50 para la actividad anticolinesterásica fue de 43,27 ± 2,22 ppm, pudiendo asociarse la actividad larvicida del extracto a su capacidad inhibitoria sobre esta enzima.
Palabras clave: actividad larvicida, extracto etanólico de marañón, mosquitos.
INTRODUCTION
The synthetic chemical insecticides are the primary option to combat adult mosquitoes and immature forms of Aedes aegypti, Anopheles sp. and Culex quinquefasciatus. These mosquitoes are vectors of human diseases such as Dengue, Chikungunya, and Zika viruses (A.aegypti), malaria (Anopheles), filariasis and viral encephalitis (C. quinquefasciatus) [1]. Brazil and Cuba are among the countries where dengue is endemic. The morbidity of dengue in Brazil grows up 48 percent until May 2016, while Zika virus is now a world pandemic. Brazil has the highest number of cases of Zika virus around the world, followed by Colombia and Venezuela [2].
It was reported the appearance of resistance of mosquitoes to some chemical insecticides like DDT (an organochloride) [3], Malathion, Diazinon and others organophosphates, especially in some populations of Brazil, Puerto Rico, and others Caribbean countries [4]. Additionally, a decrease in mosquitoes' susceptibility to the activity of the pyrethroids has been observed [5]. That is why it is urgent to find new alternatives for mosquito control, different from the classical chemical insecticides. In this sense, natural products can be an eco-friendly source of compounds with bioactivity against mosquitoes and others vectors. Many secondary metabolites have been tested as alternatives to vector control, demonstrating their larvicide activity in different ways [6]. In this context, several studies to discover vegetal substances for this approach have been performed [7].
Literature refers that some species of the Anacardiaceae family prove a larvicide activity. This family contains about 600 species distributed through 73 genera, including the genus Anacardium, which has eight species [8]. A. occidentale, popularly known as cashew (Cajú, in Brazil and Marañón in Cuba), is distributed around the world in tropical zones. The bark and leaves contain anti-inflammatory and astringent agents, which are used to prepare a decoction considered effective in the treatment of diarrhea, diabetes, eczema. It is also used as mouthwash [9].
MATERIALS AND METHODS
Plant collection: The fruit of A. occidentale was collected in the morning time at the municipality of Macapá, Amapá, Brazil (030 380N / 498 820W) in October 2015. Dr. Patrick Canterbury makes the plant identification and a voucher specimen with the register number 018781 was deposited at the Amapá Herbarium (HAMAB), Macapá, Amapá, Brazil.
Preparation of the cashew nutshell extract: The cashew nutshell was separated from the fruit and cut, longitudinally, with steel knife in order to remove the core. The cashew nutshell was washed with distilled water for mechanical separation of impurities. One gram of cashew nutshell was extracted by Maceration (72 h) in an amber vessel using a proportion of three milliliters of ethanol 70 % for each gram of drug. Every day, the extract was manually stirred. After 72 h the extract was filtered and stored at room temperature (25 ºC).
Physical properties of the extract: The pH of the 70 % hydro-ethanolic extract from cashew nutshell was measured on a pH meter mPA210 (MS TECNOPON, Brazil). The equipment was calibrated by using pH 7,0 (± 0,02) buffer solutions and pH 4,0 (± 0,02) (Alphatec, Brazil). Measurements were performed in triplicate and results were expressed as the mean and standard deviation.
Relative density was determined at 25 °C by using a pycnometer method [12]. A set of 3 pycnometers (50 mL) and an analytical balance with accuracy to 0,000 1g (Acculab, Sartorius, Germany) were used. The analysis was performed in triplicate and results were expressed as the mean and standard deviation.
The Total Soluble Substances contained in the cashew nutshell hydro-ethanolic extract were determined by gravimetric method [12] with some modifications. In a porcelain crucible, 5 mL of sample was placed and the mass was determined (CME) on an analytical balance (0,000 1g, Acculab, Sartorius, Germany). Afterwards, the crucible with the sample was placed in a water bath at 100 °C until almost the liquid present was removed. Then, the crucible was placed in an oven for 1 hour at 105 ºC. After this period, the crucible with the residue was placed in a desiccator at room temperature, to be weighed subsequently. The crucible was placed back in the oven following the same procedure and after one hour is weighed again. The process continues until two consecutive weight of the crucible with the residue (CMF) not differ more than 20 mg. The assay was performed in triplicate. The Total Soluble Substances (TS) expressed in percent was calculated using the equation 1:
Phytochemical screening: Phytochemical screening was performed to evaluate the presence of secondary metabolites by using the Chabra et al. methodology [13]. It was tested for the presence of phenols, lipids substances, quinones, alkaloids, flavonoids, triterpenes, steroids, saponins, and anthocyanidins.
Total phenols: Total phenols content was obtained by Folin-Ciocalteu method at 760 nm [14]. Calibration curve was constructed using pyrogallic acid (Sigma-Aldrich, USA) as external standard at 0,025, 0,050, 0,075, 0,100 and 0,125 mg/50 mL. A spectrophotometer QUIMIS, AA6300 (Shimadzu, Japan) was used. The expression of the calibration line was by the equation 2. Determinations were made in triplicate.
Evaluation of larvicidal activity: Fourth-stage larvae of A. aegypti (Macapá and Rockefeller strains) and Culex quinquefasciatus were used, which came from the Arthropod Laboratory (ARTHROLAB), Federal University of Amapá. The experimental protocol was performed according to the World Health Organization (WHO) [15]. One mL of the hydro-ethanolic extract to be tested was diluted in 99 mL of distilled water to form a 1 % (as total solids) homogeneous solution.
Six experimental groups (for each species) using one of them as control and the other five with a concentration of 10, 25, 50, 75, 100 ppm were performed. A total of 25 larvae per group were placed in the beaker. For the control group, in every case, 99 ml of distilled water plus 1 mL of the solvent in which were prepared the extract (ethanol 70 %) was used. All experiments were performed in triplicate and mortality was recorded after exposure periods of 24 hours.
Acetylcholinesterase (AChE) inhibitory activity: The acetylcholinesterase inhibition assay was developed as follow. An aliquot of the extract was dissolved in a base-tris (0,05 M) buffer, following the Ellman methodology as described by Niño et al. [16]. Five levels of concentration (250 ppm, 125 ppm, 75 ppm, 50 ppm and 25 ppm) were tested. Thus, 200 µL of acetylthiocholine iodide (15 mM, Sigma, USA), 1 000 µL of Ellman's Reagent, 5,5'-Dithio-bis-(2-nitrobenzoic acid) (3 mM, Sigma, USA), and 200 µL of the extract solution were placed in the test tube. The reaction mix was incubated for 25 min at 30 ºC. Then, the absorbance was measured (Shimadzu, Japan) at 412 nm. After that, 200 µL of AChE (0,3 U/mL) solution were added to start the reaction and then the absorbance was read. A mixture of all components without the extract was used as a control. The percentage of AChE inhibitory activity (% I AChE) was calculated by the equation (3):
All experiments were performed in triplicate. The IC 50 was determined by Probit analysis using the StatGraphics Centurion.
DISCUSSION OF RESULTS
Preliminary characterization of the 70 % hydro-ethanolic extract
The extract showed a characteristic odor, with a translucent appearance and homogeneous dark brown color. The presence of particles in suspensions was not observed. The physicochemical characterization of the extract is presented in table 1. The phytochemical screening detects the presence of phenolic compounds, coumarins, quinones, anthocyanidins, triterpenes, steroids, flavonoids, saponins and lipid substances. The total phenol content was calculated in 5,52 µg/mL.
TABLE 1. PHYSICOCHEMICAL CHARACTERISTICS OF THE HYDRO-ETHANOLIC EXTRACT (70 %) FROM CASHEW OF (A. occidentale) NUTSHELL
| Property | Unity | Mean ± SD |
| Density (20 °C) | g/mL | 0,910 ± 0,001 |
| pH (1/ 15 mL of distilled water) | - | 5,08 ± 0,03 |
| Total polyphenols | % | 5,52 ± 0,001 |
| Total Soluble Substances | % | 10,49 ± 0,17 |
Evaluation of larvicidal activity
The result of the larvicidal activity of cashew nutshell extract is presented in table 2. The values of LC50 and LC90 with their limits at 95 % of confidence interval are presented as well. The LC 50 and LC90 for each species tested were statistically significant (Data not shown). The R2 value of the Probit analysis was greater than 85 %, in all cases.
TABLE 2. MORTALITY LEVELS INDUCED BY THE 70 % HYDRO-ETHANOLIC EXTRACT FROM CASHEW (Anacardium occidentale L.)
NUTSHELL ON LARVAE OF C. quinquefasciatus, A. aegypti MACAPÁ, AND ROCKEFELLER STRAIN
| Specie | Group | Concentration | Mean ± SD | Larvicidal activity (95 % C.I., ppm) | Adjusted R2 | |
| (ppm) | (%) | LC50 | LC90 | |||
| A aegyptiRockefeller Strain | Control | 0 | 0,00 ± 0,00 | 35,81 | 69,77 | 85,92 |
| 1 | 10,00 | 28,00 ± 5,00 | (28,87-43,03) | (60,82-81,91) | ||
| 2 | 25,00 | 46,67 ± 7,90 |
| |||
| 3 | 50,00 | 66,67 ± 2,90 |
| |||
| 4 | 75,00 | 93,33 ± 7,90 |
| |||
| 5 | 100,00 | 100,00 ± 0,00 |
| |||
| A. aegyptiMacapá strain | Control | 0 | 0,00 ± 0,00 | 40,21 | 76,25 | 87,50 |
| 1 | 10,00 | 22,67 ± 2,90 | (34,04-44,06) | (70,60-82,91) | ||
| 2 | 25,00 | 44,00 ± 5,00 |
|
| ||
| 3 | 50,00 | 62,67 ± 2,90 |
|
| ||
| 4 | 75,00 | 92,00 ± 0,00 |
|
| ||
| 5 | 100,00 | 100,00 ± 0,00 |
|
| ||
| Culexquinquefasciatus | Control | 0 | 0,00 ± 0,00 | 21,47 | 36,05 | 90,62 |
| 1 | 10,00 | 24,00 ± 5,00 | (19,15-23,97) | (32,79-40,21) | ||
| 2 | 25,00 | 40,00 ± 5,00 |
|
| ||
| 3 | 50,00 | 56,00 ± 5,00 |
|
| ||
| 4 | 75,00 | 91,00 ± 5,00 |
|
| ||
| 5 | 100,00 | 100,00 ± 5,00 |
|
| ||
AChE inhibition activity
It was noticed a concentration/activity relationship, making possible the calculation of the IC50. The acetylcholinesterase inhibitory activity of the extract expressed as IC50 was estimated in 43,27 ± 2,22 ppm.
The extract presents a homogeneous and brilliant brown appearance. The pH value of the extract (5,08 ± 0,03) can be attributed to the presence of secondary metabolites with acidic groups (phenolic compounds, anthocyanidins, and terpenoids) as well as free fatty acid.
Total phenols content was estimated in 5,52 ± 0,01 µg/mL. This value is in concordance with the reviewed literature when signed the phenolic compounds as the most abundant metabolites in the Anacardium occidentale extracts [10]. The measured density for the extract kept a close relation with the solvent ethanol 70 %. The total soluble substance variable shows an acceptable level of the substances extracted.
Concerning the larvicidal activity of the extract in the three mosquitos larvae (adjusted R2 >85 %), it indicates that the observed mortality (Dependent variable) is plenty explained by the variation of the extract concentration (Independent variable in the Probit analysis).
The potential larvicidal application of natural products can be classified considering mortality levels of larvae after 24-48 h of treatment with concentration of 250 ppm, as follows: promising (mortality > 75 %), partially promising (50 % > mortality < 75 %), weakly promising (25 > mortality < 50 %) and inactive (mortality < 25 %) [17].
In this context, the 70 % hydro-ethanolic extract of cashew nutshell can be declared as a powerful larvicidal agent against the two mosquitoes' larvae tested with IC50 with values five times lower that what is declared as promising larvicidal. It is noticeable that a 100 % of mortality was achieved at the higher concentration tested in the three different mosquito's larvae. Therefore, this extract could have a great impact on the vector of Dengue and Zika viruses in field experiments.
The inhibition of acetylcholinesterase is one of the mechanisms involved in the insecticide and larvicidal activity of chemical and natural products [6]. A variety of plants has been reported to show AChE inhibitory activity. Almost all the metabolites detected in the 70 % hydro-ethanolic extract of cashew nutshell were reported for their AChE inhibitory activity in previous reports [18]. The IC50 estimated for this extract was 43,27±2,22 ppm. This value can be considered good if compared to a variety of natural compound reported by Suganthy et al. [18]. This fact suggests that the potent larvicidal activity of this extract could be associated with their AChE inhibitory activity as a possible action mechanism.
CONCLUSIONS
The 70 % hydro-ethanolic extract of cashew nutshell shows a great variety of chemical compound that can be responsible for the potent natural larvicidal activity demonstrated against the three mosquito larvae tested. At the light of these results, this extract could be used for larvae control of A. aegypti and C. quinquefasciatus in backyards and forest zones. It is signed also that the larvicidal activity could be related to the strong AChE inhibition. This work constitutes the first report in the chemical characterization of a 70 % hydro-ethanolic extract of the Anacardiumoccidentale L. cashew nutshell and their applicability as a potential larvicidal agent.
ACKNOWLEDGMENTS
This work is supported by CNPq process No. 402332/2013-0.The authors specially thank the support of the Universidade Federal do Amapá, Brazil, and CAPES. We want also to thanks MSc. Ricardo Marcelo dos Anjos Ferreira; Karen Santos; MSc. Ariadna Lafourcade Prada for their contributions to this paper.
REFERENCES
1. BARIK, K. T.; KAMARAJU, R.; GOWSWAMI, A. "Silica nanoparticle: a potential new insecticide for mosquito vector control". Parasitology Research. 2012, 111(3), 1075-1083. ISSN: 0932-0113
2. WHO. Dengue: Data, maps and statistics of the PAHO/WHO. Number of Reported Cases of Dengue and Severe Dengue (SD) in the Americas, by Country: Deaths (SD/D) x100 CFR North America. Epidemiological Week 11. 2016.
3. DE ANDRADE, R. P. K.; RAILDA, A. R.; ALVES, A. M.; LIMA, A. C. C.; MICHELLINE, S. E. "Insecticidal activity of the liquidof cashew nuts on Aedes aegypti (Linnaeus,1762) (Diptera: Culicidae) [In Portuguese]". Revista Brasileira de Biociências. 2013, 11(4), 419-422. ISSN: 1980-4849
4. KASAI, S. et al. "Mechanisms of Pyrethroid Resistance in the Dengue Mosquito Vector, Aedes aegypti: Target Site Insensitivity, Penetration, and Metabolism". PLoS Neglected Tropical Diseases. 2014, 8(6), e2948. ISSN: 1935-2735
5. PEREIRA DA CUNHA, M.; LIMA, B. J. P.; BROGDON, V.; MOYA, E. G.; VALLE, D. "Monitoring of resistance to the piretroids cypermethrin in Brazilian Aedes aegypti (Diptera: Culicidae) populations collected between 2001 and 2003". Memórias do Instituto Oswaldo Cruz. 2005, 100(4), 441-444. ISSN: 0074-0276
6. RATTAN, S. R. "Mechanism of action of insecticidal secondary metabolites of plant origin". Crop Protection. 2010, 29(9), 913-920. ISSN: 0261-2194
7. GUISSONI, P. A. C.; SILVA, G. I.; GERIS, R.; CUNHA, C. L.; SILVA, G. H. H. "Larvicidal activity of Anacardium occidentale as an alternative to the control of Aedes aegypti and their toxicity in Rattus norvegicus. [In Portuguese]". Revista Brasileira de Plantas Medicinais. 2013, 15(3), 363-367. ISSN: 1516-0572
8. EIJNATTEN, M. C. L. Anacardium occidentale. In: Halvey, A.H. (Editor). Handbook of flowering. First edition. CRC Press, Boca Raton, Florida: USA; 2009. ISBN: 9780849339165
9. NNAMANI, V. C.; OSAYI, E. E.; AAMA, I. C.; NWACHUKWU, C. "Larvicidal effects of leaf, bark and nutshell of Anacardium occidentale on the larvae of Anopheles gambiae in Ebonyi State, Nigeria". Animal Research International. 2011, 8(1), 1353-1358. ISSN: 1597-3115
10. HAMAD, B. F.; MUBOFU, B. E. "Potential Biological Applications of Bio-Based Anacardic Acids and Their Derivatives". International Journal of Molecular Sciences. 2011, 16(4), 8569-8590. ISSN: 1422-0067
11. FARNESI, C. L. et al. "Physiological and Morphological Aspects of Aedes aegypti Developing Larvae: Effects of the Chitin Synthesis Inhibitor Novaluron". PLoS ONE. 2012, 7(1), e30363. ISSN: 1932-6203
12. BRAZILIAN PHARMACOPOEIA. Agencia de Vigilância Sanitária, ANVISA. Brasil. 2010.
13. CHABRA, S. C.; ULSO, F. C.; MSHIU, E. N. "Phytochemical screening of Tanzanian medicinal plants (I)". Journal of Etnopharmacology. 1984, 11(2), 157-179. ISSN: 0378-8741
14. BRITISH PHARMACOPOEIA (BP). Her Majesty Stationary office. London, UK. 2010.
15. WHO. World Health Organization. Guidelines for laboratory and field-testing of mosquito larvicidal. World Health Organization Communicable disease control, prevention and eradication. WHO pesticide evaluation scheme. 2005. Geneva, Switzerland.
16. NIÑO, J.; HERNÁNDEZ, J. A.; CORREA, Y. M.; MOSQUERA, O. M. "In vitro inhibition of acetylcholinesterase by crude plant extracts from Colombian flora". Memórias do Instituto Oswaldo Cruz. 2006, 101(7), 783-785. ISSN: 0074-0276
17. MONTENEGRO, L. H. M. et al. "Terpenóides e avaliação do potencial antimalárico, larvicida, anti-radicalar e anticolinesterásico de Pouteria venosa (Sapotaceae)". Revista Brasileira de Farmacognosia. 2006, 16, 611-617. ISSN: 1981-528X
Recibido: 13/02/2017
Aceptado: 24/04/2017
Dr. C. Jesús Rafael Rodriguez Amado, Laboratório de Pesquisa em Fármacos. Faculdade de Ciências da Saúde. Universidade Federal do Amapá, Macapá, AP. Brasil, jiribilla2009@gmail.com