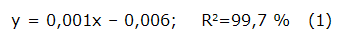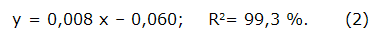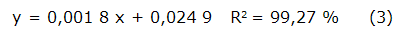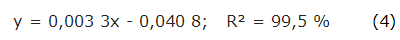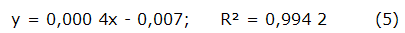Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Química
versión On-line ISSN 2224-5421
Rev Cub Quim vol.30 no.2 Santiago de Cuba may.-ago. 2018
ARTICULOS
Chemical composition and in-vitro antioxidant activity of extracts of Adelia ricinella L.
Composición química y actividad antioxidante in-vitro de extractos de Adelia ricinella L.
MSc. Clara Azalea Berenguer-RivasI, Lic. Mariuska Mas-OrtizI, MSc. Pedro L. Batista-CorbalII, Lic. Jainer Costa-AcostaIII, Dr. C Julio César Escalona-ArranzI
IPharmacy Department, Universidad de Oriente, Cuba, clarabr@uo.edu.cu, jcea@uo.edu.cu
IIToxicology and Biomedicine Center, TOXIMED, Santiago de Cuba, Cuba
IIIEastern Center for Ecosystems and Biodiversity (BIOECO), Santiago de Cuba, Cuba
ABSTRACT
Adelia ricinella L. is a plant used as analgesic, antipyretic and emenagogue. In this research, three extracts from the aerial parts of the plant were prepared by Soxhlet extraction, using 95 % ethanol, 50 % and water as solvents. The main secondary metabolites were determined from qualitative and quantitative points of view. Antioxidant activity was measured using in-vitro radical scavenging methods facing ABTS and DPPH radicals. The qualitative analysis shows alkaloids, coumarins, reducing sugars, phenols, carbohydrates and flavonoids, quantifying a higher content of the last four metabolites in the ethanol 50 % extract. Similarly, the 50 % ethanol extract was the most active, determining an average inhibitory concentration (IC50) of 0,29 ± 0,01 mg/mL against ABTS and 0,53 ± 0,02 mg/mL when faced to DPPH. These evidences show that extract prepared from A. ricinella could be useful for the treatment of diseases related by oxidative stress.
Keywords: Adelia ricinella L., chemical composition, total phenols, flavonoids, antioxidant activity.
RESUMEN
Adelia ricinella L. es una planta empleada por la población como analgésico, antipirético y emenagogo. En la presente investigación se obtuvieron tres extractos por extracción con Soxhlet, empleando etanol 95 %, 50 % y agua como solventes, a partir de las partes aéreas de la planta. A los extractos se le determinaron los principales metabolitos secundarios cualitativa y cuantitativamente. La actividad antioxidante fue medida empleando métodos in–vitro de neutralización de los radicales ABTS y DPPH. El análisis cualitativo mostró la presencia de alcaloides, cumarinas, azúcares reductores, fenoles, carbohidratos y flavonoides, cuantificándose un mayor contenido de los cuatro últimos en el extracto etanol 50 %. De igual forma, el extracto etanólico al 50 % fue el de mayor actividad, determinándose una concentración inhibitoria media (CI 50 ) de 0,29 ± 0,01 mg/mL para ABTS y 0,53 ± 0,02 mg/mL para DPPH . Las evidencias obtenidas demuestran que la A. ricinella pudiera resultar útil para el tratamiento de enfermedades causadas por el estrés oxidativo.
Palabras clave: Adelia ricinella L, composición química, fenoles totales, flavonoides, actividad antioxidante.
INTRODUCTION
In the progressive development of medicine as a science, there has been a resurgence of Natural and Traditional Medicine (NTM). The use of herbal medicines and supplements has increased enormously in recent years, demonstrating since the late 80´s; the preference of the natural products in detriment of synthetic products in all market segments [1, 2].
The botanical family Euphorbiaceae , is one of the largest families in the world occupying the sixth place in diversity and extension. It consists in 300 genus and around 7 500 species; mainly distributed in the tropics, specifically in the Indo-Malay and tropical America regions.
From the chemical point of view, reports from more than 120 species of this family appears in the scientific literature, demonstrating the presence of secondary metabolites such as terpenes, amino acids, quinones, saponins, tannins, alkaloids and phenolic compounds (flavonoids, lignans, coumarins, tannins, quinones, phenolic acid, etc.) [3-6]. Terpenoid compounds such as angelic acid, 7-Hydroxy-1,6cyclo-2,10,14-phytatrien-4-one and Vomifoliol are reported for this family. Alkaloids of the aporphinic type, quinolines and tropane like: Crotonosine, Hernovine, Jacularine, Nuciferine, Salutaridine, Wilsonirine, Crotonoside and Linearisine and phenolic compounds like: flavones, flavonoids glycosides, kaenferol, myricetina and gallactocatechine are also some of the isolated compounds in this family. Unsaturated fatty acids are present too [7, 8]. This chemical composition it has conferred a wide range of therapeutic applications such as immunomodulatory, anti-inflammatory, analgesic, antimicrobial used in the treatment of tumors, in some types of cancer, inflammations, asthma, fever, skin diseases, pneumonia, insecticide, rheumatic pain among others [3, 5-8].
Other researchers have emphasized the evaluation of the antioxidant activity of different species belonging to the family Euphorbiaceae, since the search and obtaining natural antioxidants to replace the synthetic ones, it has become a subject of high interest in the biological, medicinal, nutritional and agrochemical fields [3, 9-12].
Adelia ricinella L. better known as "Jía Blanca", is a species of tree belonging to the family Euphorbiaceae [13]. It is native to the Caribbean, in Cuba is located throughout the archipelago and Isla de la Juventud, also inhabits regions of Venezuela and Colombia, where medicinal properties are attributed as antipyretic, analgesic, abortive and anti-inflammatory [14, 15]. From the scientific point of view, there is short information about this plant, however; several ethno-pharmacological uses are reported by the population. This fact demonstrates the need to carry out a deeper study of its chemical composition and pharmacological potentialities. Therefore, the following work we aimed to determine and quantify the main secondary metabolites of extracts of the aerial parts of Adelia ricinella L. and to evaluate its antioxidant properties.
MATERIALS AND METHODS
The aerial parts of the Adelia ricinella L. plant were collected in March 2017 in the Siboney-Juticí Ecological Reserve, in the municipality of Santiago de Cuba. A plant sample was taxonomically identified by specialist at the Eastern Center for Ecosystems and Biodiversity (BIOECO) in the province of Santiago de Cuba and a vegetal sample was settled at the herbarium of said institution with the registration number 14 780.
Extracts Preparation
The extracts were prepared by Soxhlet extraction letting four hours after the first reflux using water, ethanol 50 % and ethanol 95 %, obtaining three extracts. Once the extracts were obtained were filtered using a Buchner funnel and filter paper they were concentrated in a KIRKA - WERKE Rotary Evaporator (Germany) reducing the final volume to 100 ml, obtaining final concentrations equivalent to 10 mg/mL (dry plant material weight).
Physical-chemical characterization of the extracts obtained
The physical-chemical characterization of the extracts was carried out according to the established by the Cuban National Standards defined by the Health Ministry [16, 17]. The parameters determined were:
- Organoleptics characteristics: organoleptic properties were evaluated by simple inspection through the senses. Colour, texture and smell were evaluated.
- pH: A direct pH value was obtained from a pH-meter (Hanna Instruments, Spain). The pH-meter was calibrated using buffer solutions at pH 4 and 7. Three measurements were fulfilled and the mean was reported
- Relative density: was determined using a 25 mL picnometer. Three measurements were fulfilled and the mean was reported.
- Total solids: were determined using a Gravimetric method with 5 mL in a porcelain capsule.
Qualitative chemical composition determination
Chemical reactions established in the phytochemical screening technique described in the literature were performed to define the metabolites or groups of metabolites present in the plant under study [18]. The metabolites to be determined were Alkaloids, Amino Acids and Amines, Carbohydrates, Saponins, Phenols and Tannins, Reducing Sugars, Triterpenes and Steroids, Quinones, Coumarins, and Flavonoids. In brief:
1. Test for Alkaloids
a. Mayer's test: add to one mL of plant sample extract two drops of Mayer's reagent. Appearance of white creamy precipitate indicates the presence of alkaloids.
b. Wagner's test: a few drops of Wagner's reagent are added to one mL of plant extract. A reddish- Brown precipitate confirms the test as positive.
2. Test for Amino acids
a. Ninhydrin test: two drops of ninhydrin solution (10 mg of ninhydrin in 200 ml of acetone) were added to 2 ml of aqueous filtrate extract. The appearance of a purple colour indicates the presence of amino acids.
3. Test for Carbohydrates
a. Molish' s test: to 2 ml of plant sample extract, two drops of alcoholic solution of a - naphthol were added. The mixture was shaken well and few drops of concentrated sulphuric acid was gently added. A violet ring indicates the presence of carbohydrates.
4. Test for Saponins
a. Foam Test: the extract (10 mL) was diluted with 10 mL of distilled water. The solution was shaken for 15 minutes. A two cm layer of foam indicates the presence of saponins.
5. Test for Phenolic compounds and Tannins
a. Ferric Chloride test: to 1 mL of the extract were added few drops of neutral 5 % ferric chloride solution. A dark green colour indicates the presence of phenolic compound.
6. Test for reducing sugars
a. Benedict's Test: two mL of the extract were treated with Benedict's reagent and heated gently. Orange red precipitate indicates the presence of reducing sugars.
b. Fehling's Test: two mL of the extract were hydrolyzed with dil. HCl, neutralized with alkali and heated with Fehling's A & B solutions. Formation of red precipitate indicates the presence of reducing sugars.
7. Test for Triterpenos
a. Salkowski's Test: two mL of the extract dried and re-dissolved in chloroform and filtered. The filtrates were treated with few drops of concentrated Sulphuric acid, shaken and allowing to stand. Appearance of golden yellow colour indicates the presence of triterpenes.
b. Libermann Burchard's test: two mL of the extract dried and re-dissolved in chloroform and filtered. The filtrates were treated with few drops of acetic anhydride, boiled and cooled. Concentrated Sulphuric acid was added.
8. Test for Quinones
a. Borntrager's Test: take 1 mL of the extract, concentrated until drieness and re-dissolved in 1 ml of benzene. One millilitres of the 10 % ammonia solution was then added and shaked. Appearance of a pink, red or violet colour in the ammoniacal (lower) phase was taken as the presence of free quinones.
9. Coumarins test
a. Baljet test: one milliliter of the extract was treated with a solution of sodium picrate. The formation of a yellow colour indicates the presence of lactones.
10. Test for flavonoids
a. Concentrated sulfuric acid: to one milliliter of the extract were added in a test tube 2 mL of dilute ammonia solution. After this, few drops of concentrated H2S04 were added. A yellow colour indicates the presence of flavonoids.
b. Shinoda's test for flavonoids: one mililiter of the extract is dried and re-disolved in 1 mL ethanol. Three pieces of magnesium chips was then added to the filtrate followed by few drops of conc. HCl. A pink, orange, or red to purple colouration indicates the presence of flavonoids
c. Alkaline Reagent Test: extracts were treated with few drops of sodium hydroxide solution. Formation of intense yellow colour, which becomes colourless on addition of dilute acid, indicates the presence of flavonoids.
Quantification of Total Phenolic content (TPC)
The total phenolic content was determined by the colorimetric method using Folin-Ciocalteu's reagent (Sigma, USA) [19]. The reagent reacts with phenol groups by a REDOX reaction getting a blue colour. The colour produced is proportional to the amount of polyphenols present in the extract analyzed, absorbing from 750 to 770 nm. Briefly, 500 µ L of sample (1 mg/mL solution based on total solids) was mixed with 1 mL of 50 % Folin-Ciocalteu's and incubated at room temperature in a dark room. Subsequently, 2 mL of saturated sodium carbonate solution (Riedel-de Haën, 99,5 % pure, Germany) was added. Finally, the solution was incubated at room temperature in the darkness for one hour. The absorbance was measured at 765 nm on a UV/VIS spectrophotometer from PG Instruments, model T60 (China). A standard curve using gallic acid (GA) (Sigma, USA) was created with 7 points (from 9,7 to 625 µ g/mL). The mathematical equation used to calculate the concentration was:
The results were expressed as mg in gallic acid equivalents /g extract. All measurements are repeated 3 times. Based on findings of Dudonne et al., TPC were categorized as very high (> 300 mg GAE/g), high (200 - 300 mg GAE/g), moderate (50 - 200 mg GAE/g), low (15 - 50 mg GAE/g), very low (< 15 mg GAE/g) [20].
Quantification of Total Flavonoid Content (TFC)
The quantification of the total flavonoid content was assessed through reaction with aluminum trichloride (AlCl3, Riedel-de Haën, 99,9 % pure, Germany) [21]. Briefly, 250 µL of the extracts (1 mg/mL solution based on total solids) were mixed with 1,25 mL of distilled water and 75 µL of a 5 % NaNO2 solution. After five minutes, 150 µ L of a 10 % AlCl3 aqueous solution was added. After six minutes, 500 µL of 1M NaOH and 275 µL of distilled water were added. The solution was mixed well and read at 510 nm in the mentioned spectrophotometer.
Values were determined from a calibration curve prepared with quercetin (Q) (Sigma, 95 % pure, USA) (ranging from 6,25 to 100 µ g/mL) and expressed as mg of quercetin equivalent /g extract. The mathematical equation used to calculate the concentration of the sample expressed as quercetin was:
Considering the previous criteria, TFC were categorized as very high (>300 mg QE/g), high (200-300 mg QE/g), moderate (50 - 200 mg QE/g), low (15 - 50 mg QE/g), very low (< 15 mg QE/g) [20].
Quantification of Total Protein Content
The quantification of total proteins was performed following the Lowry methodology [22]. One milliliter of the samples (1 mg/mL solution based on total solids) were added to 5 mL of the Lowry reagent, which is composed of three solutions mixed at the time of use: 2 % sodium carbonate in 0,1 M NaOH (50 mL), 1 % cupric sulfate (0,5 mL) and 2 % sodium potassium tartrate (0.5 mL). After 15 min, 0,5 mL of Folin-Ciocalteu reagent was added, allowing the mixture to stand for another 30 min. At the end, absorbance was measured at 595 nm. A standard curve was prepared using Bovine Serum Albumin (BSA) in a concentration range between 5 - 200 µ g/mL. The mathematical equation that describes it behavior is reflected in equation 3. The results were expressed as g BSA equivalent /100 g extract.
Quantification of Carbohydrate
The quantification of carbohydrates was realized by the phenol-sulfuric method, according to the methodology described by Dubois [23]. Two milliliter of the samples (1 mg/mL solution based on total solids) were mixed with 2 mL of 5 % phenol in test tubes and placed in a rack submerged in a cold water bath. Five milliliters of H2SO4 were added letting the tubes for 15 min to measure the absorbance at 490 nm in a UV/VIS spectrophotometer. Glucose (Riedel-de Haën, 99,5 % pure, Germany) was used for the calibration curve in concentrations from 10 to 200 µ g/mL, generating the following equation:
All measurements are repeated 3 times. The results were expressed as g of glucose equivalent /100 g extract.
Quantification of Total reducing sugars
The determination of total reducing sugars was done using the 3,5-dinitrosalicylic acid (DNS,Sigma-Aldrich, 98 % pure, USA), following the methodology described by Miller [24]. For the preparation of this reagent, 0,8 g of NaOH (Riedel-de Haen, 97 % pure, Germany) was dissolved in distilled water, then 15 g of potassium sodium tartrate tetrahydrate (Fluka, 99 % pure, Germany) was added as well as 0,5 g of DNS. This mixture was poured into 50 mL with distilled water and stored in an amber flask at 4 °C. Subsequently 0,5 mL of each sample (1 mg/mL solution based on total solids) and 0,5 mL of the DNS reagent were placed in a beaker, boiled for 5 min to further stopthe reaction adding cold water/ice. Five milliliters of distilled water were added to the samples, shaken and rested for 15 min. The absorbance was determined at 540 nm in spectrophotometer (T60 UV-Visible Spectrophotometer). The same treatment was performed for the blank with distilled water. A calibration curve was developed, using glucose as standard in concentrations from 0,1 to 1 mg/mL, obtaining the following calibration curve:
All measurements are repeated 3 times. The results were expressed as g glucose equivalent /100 g extract.
Evaluation of antioxidant activity
Antioxidant activity against the radical 2,2-azino-bis- (3-ethyl benzothiazolin-6-sulfonic acid) (ABTS).
It was developed according to the methodology described in the literature [25]. The assay is based on the ability of an antioxidant compound to quench the ABTS radical (Sigma-Aldrich, 98 % pure, USA). To reach that goal, 50 µ L of each extract (solutions of 62,5, 125, 250, 500 and 1 000 µ g/mL based on total solids) were added to 3 mL of diluted ABTS solution and after 90 min the absorbance was measured at 734 nm. A solution of 50 µ L of distilled water and 3 mL of diluted ABTS was used as absorbance blank. An ascorbic acid (Fluka, 99 % pure, Germany) at a concentration of (1 mg/mL) was considered as positive control. The ability of radical quench was determined by calculating the percent inhibition of the radical (IpABTS) according to the formula:
The scavenge of ABTS radicals by the extracts of Adelia ricinella L. were estimated as a function of the concentrations of extracts capable of quench the 50 % of the radical (IC 50) obtained by intrapolation in the curve constructed from the five concentrations evaluated. All the experiments were repeated three times.
Antioxidant activity against the radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH)
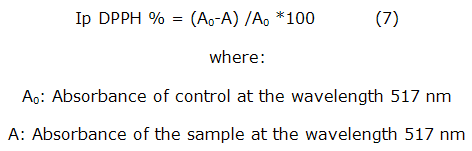
It was followed the standard methods described in the literature [26]. In brief: A solution of 0.1 mM of DPPH (MERCK, pure for analysis, USA) was prepared using 0,003 94 g dissolved in 100 mL of ethanol. A total of 0,25 mL of the extracts (solutions of 62,5, 125, 250, 500 and 1 000 µ g/mL based on total solids) were placed in test tubes, where were added 1,5 mL of the DPPH solution. The mix was shaken in a vortex (Heidolph REAX 2000, Germany) and kept in the dark for 20 min. The absorbance was measured in spectrophotometer (T60 UV-Visible Spectrophotometer) at 517 nm. The absorbance blank was prepared with 0,25 mL of ethanol and 1,5 mL DPPH solution.The positive control was an ascorbic acid solution a concentration of 1 mg/mL. The radical sequestration ability was determined by calculating the percent inhibition of the radical (Ip DPPH) by the equation:
In addition, the antioxidant capacity against these radicals was estimated as a function of the concentrations of the extracts at 50 % inhibition (IC50) obtained by intrapolation in the calibration curve constructed with the five concentrations evaluated. All the experiments were repeated 3 times.
Statistic analysis
For the statistical analysis, Microsoft Excel was used (Microsoft Office 2007 package) as well as STATGRAPHICS Plus Version 5.1 software. The results of Total phenols, flavonoids, proteins, carbohydrates and reducing sugar contents were expressed as mean ± standard deviation of each extract and their means were compared using an ANOVA Variance Analysis, aided by Statgraphic Centurion. The differences between extracts were determined by Tukey's Low Significant Differences Test (LSD). Same processing received the biological variables determined: antioxidant activities against ABTS and DPPH radicals. In all cases, the 95 % confidence limit was considered. Calibration curve equations were obtained using the Simple Linear Regression option of the STATGRAPHICS software used.
RESULTS Y DISCUSSION
The plants of the family Euphorbiaceae are present in all types of habitats, adapting to abiotic stress factors such as: high temperature, salinity and long periods of drought. This induces them to produce a variety of secondary metabolites (polyphenols, flavonoids, tannins, alkaloids, coumarins, among others) to be used for the survival and/or defense of biotic and abiotic aggressions. This varied secondary metabolism confers in turn a greater complexity and a high investigative potential, especially from the chemical and pharmacological point of view. Considering the above explained and according to the scarce information existing in the scientific literature of the species Adelia ricinella L ., it was decided to determine the quality control of the three extracts obtained in order to standardize the information. The parameters considered with standardization purposes were their physicochemical parameters (table 1).
When analyzing the organoleptic requirements, the color in the extract in 95 % ethanol resulted in an intense green hue. The extract obtained with ethanol 50 % took a light green coloration, while the aqueous extract was light brown. The three extracts are slightly viscous and have a similar odor resulting from the characteristic one for the plant material.
TABLE 1. PHYSICAL-CHEMICAL PARAMETERS EVALUATED TO THE EXTRACTS
OF THE PLANT Adelia ricinella L.
| Parameters | Ethanol | Ethanol | Aqueous extract |
| Total solids (g/100 ml) | 12,0 ± 0,28a | 5,0 ± 0,08c | 8,2 ± 0,20b |
| Relative density (g/ml) | 0,951 3 ± 0,020c | 1,0136 ± 0,008b | 1,039 3 ± 0,004a |
| pH | 4,47 ± 0,006b | 4,38 ± 0,006c | 4,70 ± 0,010a |
Different letters indicate significant differences (p < 0,05)
The values of total solids obtained indicate that the extraction method and the solvents used are suitable for the extraction of the plant metabolites. The 95 % ethanol resulted as the extraction solvent with highest amount of extracted substance expressed in mass units. This shows how solvents with medium polarity as ethanol, are suitable for the extraction of total metabolites of the species Adelia ricinella L.
Relative density results show that the aqueous extract exhibit the higher values, followed by ethanol 50 % and ethanol 95 % extracts. Those values are higher than the density of the pure solvents itself, corroborating the presence of metabolites extracted from the plant material; and are in agreement with the density of the pure solvents used. The pH values ranged from 4,38 to 4,70, being the more acidic extract the prepared with ethanol 50 %. All those physical-chemical parameters are statistically different in the three extract tested.
In table 2 it is revealed the presence of alkaloids, coumarins, reducing sugars, flavonoids, phenols and tannins, carbohydrates, amino acids and amines in the three prepared extracts. However, in the case of saponins, they were only identified in the aqueous and 50% ethanol extracts. Many of those metabolites are phenol type, probably causing the weakly acidic pH detected in the physical-chemical characterization.
TABLE QUALITATIVE CHEMICAL COMPOSITION OF EXTRACTS OF THE PLANT Adelia ricinella L.
| Metabolites | Test | Evidence | ||
| Ethanol Ext. | Aqueous Ext. | |||
| 95 % | 50 % | |||
| Alkaloids | Mayer | +++ | +++ | +++ |
| Wagner | + | + | + | |
| Triterpenes and steroids | Solkowski | + | + | N/R |
| Lieberman-Burchard | + | - | N/R | |
| Quinones | Borntrager | - | + | N/R |
| Variant with benzene | - | + | N/R | |
| Cumarins | Baljet | + | + | N/R |
| Saponins | Foam test | - | + | + |
| Reducing sugars | Fehling | + | + | + |
| Benedict | + | + | + | |
| Phenols and tannins | Ferric chloride | + | + | + |
| Free amino acids and amines | Ninhydrin. | + | + | + |
| Carbohydrates | Molisch | + | + | + |
| Flavonoids | Concentrated sulfuric acid | + | + | + |
| Shinoda | + | + | + | |
| Alkalis | - | + | + | |
Legend: (+) indicates positive evidence, (+++) indicates markedly positive evidence,
- indicates negative result; NR corresponds to unrealized tests.
The presence of alkaloids in the three extracts is quite interesting because they are compounds with a wide variety of structure and, in turn, a wide range of applications and biological activities [3, 26]. According to the colours developed in the different chemical tests, the flavonoids type present in the extracts belongs to two main subfamilies: flavones and flavonols. In ethanol 95 % extract, the presence of triterpenes and steroids with unsaturated androstane nucleus in ring B and with double bonds in carbons 5 and 6 are detected, as judged by the stains obtained in the Lieberman-Burchard reagent test. In general, these results are in correspondence with those reported in the literature for plants belonging to the family Euphorbiaceae [3, 6].
The concentration values determined for each one of the quantified metabolites are shown in table 3. It can be observed significant differences (p < 0,05) in the content of total phenols, flavonoids, carbohydrates and reducing sugars when comparing ethanolic and aqueous extracts, noting that the highest concentrations of these metabolites are found in the extract in ethanol 50 %. The Total Phenol Content for the three extracts can be considered as Very High, while total flavonoids content qualify as low (Aqueous and Ethanol 50 % extracts) and very low (Ethanol 95 % extract) according to the criteria of Dudonne [20]. These values obtained from total phenols and flavonoids are higher than those obtained in other studies of plants belonging to the family Euphorbiaceae [27 - 29].
The behavior of the protein content does not follow the same rule marked by the rest metabolites. In this case the highest concentration are found in the aqueous extract, as can be expected when is considered the high water solubility of this kind of compounds.
TABLE 3. QUANTIFICATION OF TOTAL PHENOLS, TOTAL FLAVONOIDS, PROTEINS, CARBOHYDRATES AND REDUCING
SUGARS PRESENT IN THE EXTRACTS OF Adelia ricinella L.
| Extracts
| Total Phenols | Total Flavonoids | Proteins | Carbohydrates | Reducing Sugars (g/100 g) |
| Media ± D.E | |||||
| Aqueous | 523,1 ± 12,4b | 17,33 ± 2,85b | 34,55 ± 0,64c | 49,49 ± 4,53b | 52,58 ± 3,25b |
| Ethanol 50 % | 967,9 ± 71,8c | 19,29 ± 1,09c | 24,87 ± 1,26b | 77,17 ± 18,05c | 80,91 ± 3,66c |
| Ethanol 95% | 402,3 ± 19,5a | 10,00 ± 1,00a | 10,35 ± 0,12a | 38,75 ± 4,28a | 17,67 ± 2,4a |
Different letters indicate significant differences (p < 0,05)
Evaluation of antioxidant activity
Antioxidant activity against the radical ABTS and DPPH
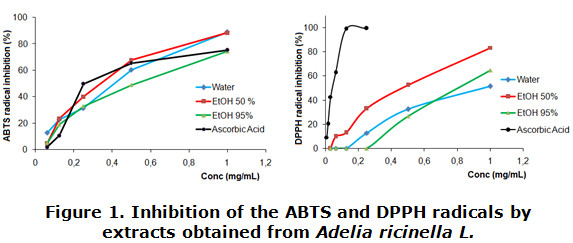
The figure 1 shows the percentage inhibition of the ABTS and DPPH radicals of the three extracts tested, generally observing how all extracts were able to neutralize these radicals in a concentration-dependent manner. When facing ABTS radical extracts had a behavior comparable to the one shown by the reference antioxidant (ascorbic acid), but not in the case of the DPPH radical, where the extracts evaluated were smaller than the positive control, exhibiting significant differences between them (p < 0,05).
Several authors have reported high antioxidant activity in different species belonging to the family Euphorbiaceae; associating this fact to the presence of a high content of phenolic compounds. However, it's possible these antioxidant properties are expressed due to the presence of other metabolites in the extracts such as alkaloids and carbohydrates [30 - 32].
Alkaloids derived from benzylisoquinoline have shown their antioxidant capacity (AC) related to the presence of an adjacent benzylic hydrogen (ortho position) to a nitrogen atom with two unpaired electrons [33]. This type of alkaloid has been frequently identified in species of the family Euphorbiaceae [34]. Additionally, reducing sugars and carbohydrates are substances produced in the primary metabolism of plants which are also reported to play important pharmacological activities. They have a carbonyl group which can act as a reductant against other molecules. Recent studies have shown the ability of protein and carbohydrate to neutralize superoxides and hydroxyl radicals; correlating with their concentrations [35].
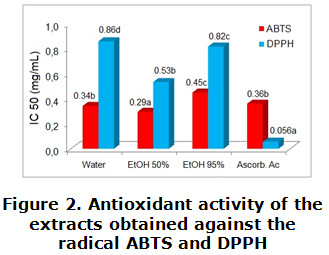
In figure 2 it is shown the extract concentration needed to decrease the initial concentration of the ABTS and DPPH radical expressed as IC 50 (mg/mL) by 50 %. Observing how in case of ABTS the results varied between 0,29 ± 0,01 to 0,45 ± 0,05 mg/mL. The extract in 50 % ethanol exhibit the lowest IC 50 with a value of 0,29 ± 0,01 mg/mL, lesser than the obtained by the substance used as antioxidant reference (ascorbic acid 0,36 ± 0,01 mg/mL). For the activity against DPPH radical the IC 50 values showed the extract in 50 % ethanol as the most active with an estimated value of IC50=0,53 ± 0,02 mg/mL, followed by the 95 % ethanolic extract (0,82 ± 0,07 mg/mL). However, this value classified as slightly higher when compared with the reference substance showing statistically significant differences (p < 0,05).
Different letters indicate significant differences (p < 0,05)
Regarding to the evaluation of the antioxidant activity of the extracts, the best results are observed in the neutralization of the radical ABTS; a method which allows to measure the activity of hydrophilic and lipophilic compounds. This method is applicable to any substance with an oxide-reduction potential thermodynamically lower than ABTS (0,68 V) acting in this way as a reductant. Another difference between the two methods is related with the maximum absorbance peaks. The ABTS radical´s spectrum shows maximum absorbance at 414, 654, 754 and 815 nm in ethanol, while DPPH presents a single peak of absorbance at 515 nm, limiting in this way the radical detection [36, 37].
CONCLUSIONS
The experimental evidence obtained shows a high antioxidant potential from the extracts of the plant Adelia ricinella L., especially the 50 % ethanol extract; even when no effective correlations with the content of total phenols, flavonoids, carbohydrates, proteins and reducing sugars is found. In consequence, the alkaloids identified through qualitative tests on the extracts, are proposed as other kind of metabolites which can contribute to the antioxidant activity evaluated. This suggestion is based on their ability to donate electrons and, therefore; to neutralize radicals. By this way, it is hypothesized a synergistic action to reach the antioxidant activity demonstrated in the extracts of this plant.
REFERENCES
1. MINISTERIO DE SALUD PÚBLICA. Programa para el desarrollo y la generalización de la Medicina Tradicional y Natural. La Habana: MINSAP, 2010.
2. SHARAPIN, N.; MACHADO, L.; ALBUQUERQUE, E.; VALVERDE, E. Fundamentos de tecnología de productos fitoterapéuticos. Sta. Fé de Bogotá. Colombia: Ed. Convenio Andrés Bello y Red Iberoamericana de Productos Fitofarmacéuticos (RIPROFITO) del Subprograma X de CYTED, 2000.
3. MWINE, J.; VAN, P. "Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features". Journal of Medicinal Plants Research. 2011, 5(5), 652–662. ISSN 1996-0875
4. DASARI, NP. "Quantification of phytochemical constituents and in vitro antioxidant activity of Synadium grantii". Free Radicals and Antioxidants. 2012, 2(2), 68–72. ISSN 2231-2536
5. BUSSMANN, W.; SHARON, D. "Traditional medicinal plant use in Northern Peru: tracking two thousand years of healing culture". Journal of Ethnobiology and Ethnomedicine. 2006, 2, 47. doi:10.1186/1746-4269-2-47. ISSN 1746-4269
6. BHASKARA, R.; KARTHIK, L.; ELUMALAI, E.; SRINIVASAN, K.; GAURAV, K. "Antibacterial and antifungal activity of Euphorbiahirta. Leaves: a comparativestudy". Journal of Pharmacy Research. 2010, 3(3), 548–549. ISSN 0974-6943
7. COY, C.; GÓMEZ, D.; CASTIBLANCO, F. "Importancia medicinal del género Croton (Euphorbiaceae)". Revista Cubana de Plantas Medicinales. 2016, 21(2), 234–247. ISSN 1028-4796
8. GARCÍA, J.; ESCALONA, J. C.; DO CARVALLOII, M. G.; ROJAS, J.; MACHADO, R.; DE LA VEGA, J. "Aislamiento y caracterización de metabolitos de hojas de Croton linearis Jacq". Revista Cubana de Química 2015, 27(3), 289–301. ISSN 2224-5421
9. SHAHWAR, D.; SUR-REHMA, N.; AHMAD, N.; ULLAH, S.; RAZA, M. A. "Antioxidant activities of the selected plants from the family Euphorbiaceae, Lauraceae, Malvaceae and Balsaminaceae". African Journal of Biotechnology. 2010, 9(7), 1086–1096. ISSN 1684-5315
10. THAMBIRAJ, J.; PAULSAMY, S.; SEVUKAPERUMAL, R. "Evaluation of in vitro antioxidant activity in the traditional medicinal shrub of western districts of Tamilnadu, India, Acalypha fruticosa Forssk. (Euphorbiaceae)". Asian Pacific Journal of Tropical Biomedicine. 2012, 2(1), 127–130. ISSN 2221-1691
11. MOSQUERA, M.; CORREA, M.; BUITRAGO, C.; JAIME, N. "Antioxidant activity of twenty-five plants from Colombian biodiversity". Memórias do Instituto Oswaldo Cruz. 2007, 102(5), 631–634. ISSN 1678-8060
12. TSUMBU, C. N.; DEBY DUPONT, G.; TITS, M.; ANGENOT, L.; FRANCK, T.; SERTEYN, D.; MOUITHYS, A. "Antioxidant and Antiradical Activities of Manihot esculenta Crantz (Euphorbiaceae) Leaves and Other Selected Tropical Green Vegetables Investigated on Lipoperoxidation and Phorbol-12-myristate-13-acetate (PMA) Activated Monocytes". Nutrients. 2011, 3(9), 818–838. ISSN 2072-6643
13. NOVA, J.; SOSA, V. "A Synopsis of Adelia (Euphorbiaceae). The American Society of Plant Taxonomists". Systematic Botany. 2007, 32(3), 583–595. ISSN 1548-2324
14. FALCÓN, A.; JUNCO, J.Z.; DOMÍNGUEZ, A.; BLANDARIZ, S.; ROSA, R. "Flora y vegetación de Lomas de La Canoa, Reserva de la Biosfera Buenavista". Revista Cubana de Ciencias Forestales. 2015, 3(1), 57–71. ISSN 2310-3469
15. BRUSSELL, D. "A medicinal plant collection from Montserrat, West Indies. The New York botanical garden press". Economic Botany. 2004, 58(1), 203–220. ISSN 0013-0001
16. MINISTERIO DE SALUD PÚBLICA. NRSP No. 309. Medicamentos de origen vegetal: droga cruda. Métodos de ensayos. La Habana: MINSAP, 1992.
17. MINISTERIO DE SALUD PÚBLICA. NRSP No. 310. Medicamentos de origen vegetal: droga cruda. Métodos de ensayos. La Habana: MINSAP, 1992.
18. OCHOA, A. P.; LÓPEZ, T. G.; COLOMBAT, M. R. Farmacognosia y química de los productos naturales. Monografía. Editado en CD-ROM ISBN, 2002, p. 959 – 207.
19. GHIMERAY, A.; JIN, C.; GHIMIRE, B.; CHO D. "Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta Indica A. Juss grown in foothills of Nepal". African Journal of Biotechnology. 2009, 8(13), 3084–3091. ISSN 1684-5315
20. DUDONNE, S.; VITRAC, X.; COUTIERE, P.; WOILLEZ, M.; MERILLON, J. M. "Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays". Journal Agricultural Food Chemistry. 2009, 57(5), 1768–1774. ISSN 0021-8561
21. JIA, Z.; TANG, M.; WU, J. "The determination of flavonoid contents in mulberry and theirs scavenging effects on superoxide radicals". Food Chemistry. 1999, 64(4), 555–559. ISSN 0308-8146
22. LOWRY, O.; ROSENBROUGH, N.; FARR, A.; RANDALL, R. "Protein measurement with the Folin phenol reagent". Journal of Biological Chemistry. 1951, 193(1), 265–275. ISSN 1083-351X
23. DUBOIS, M.; GILLES, K., HAMILTON, J.; ROBERS, P.; SMITH, F. "Colorimetric method for the determination of sugars and related substances". Analytical. Biochemistry. 1956, 28(3), 350–356. ISSN 0003-2697
24. MILLER, G. "Use of dinitrosalicylicacid re agent for determination of reducing sugar". Analytical. Chemistry. 1959, 31(3), 426–428. ISSN 1520-6882
25. CHOI, Y.; LEE, J.; CHU, H. "Influence of the heat treatment on the antioxidant activities and polyphenolic compounds of shiitake (Lentinusedodes) mushroom". Food Chemistry. 2006, 99(2), 381–387. ISSN 0308-8146
26. SHIMADA, K.; FUJIKAWA, K.; YAHARA, K.; NAKAMURA, T. "Antioxidative properties of xanthan on the autioxidation of soybean oil in cyclodextrin emulsion". Journal Agricultural Food Chemistry. 1992, 40(6), 945–948. ISSN: 0021-8561
27. YIN, B. et al. "Chemical Constituents of Plants from the Genus Excoecaria". Chemistry and Biodiversity. 2008, 5(11), 2356 – 2371. ISSN 1612-1880
28. KOUAKOU SIRANSY, G. et al. "Oxygen species scavenger activities and phenolic contents of four West African plants". Food Chemistry. 2010, 118(2), 430–435. ISSN 0308-8146
29. KUMARON, A.; KARUNAKURAN, J. " In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India". Food science and technology. 2007, 40(2), 344–352. ISSN 0975-8402
30. SUBHAN, N.; ALAM, M. A.; AHMED, F.; AWAL, M. A.; NAHAR, L.; SARKAR, S. D. "In vitro antioxidant property of the extract of Excocaria agallocha (Euphorbiaceae)". Daru. 2008, 16(3), 149–154. ISSN 2008-2231
31. KOFFUOR, G.; AMOATENG, P. "Antioxidant and anticoagulant properties of Phyllanthus fraternus GL. Webster (Family: Euphorbiaceae)". Journal of Pharmacology and Toxicology. 2011, 6(7), 624–636. ISSN 2152-100x
32. NITHYA, T.; JAYANTHI, J.; RAGUNATHAN, M. "Antioxidant activity, total phenol, flavonoid, alkaloid, tannin, and saponin contents of leaf extracts of Salvinia molesta D. S. Mitchell". Asian journal of Pharmaceutical and clinical research. 2016, 9(1), 200-203. ISSN 2455-3891
33. BRUCE, K.; ASENCIOT, M.; SPEISKY, H.; VIDELAS, L.; LISSIII, E. "Structure-antioxidative activity relationships inbenzylisoquinoline alkaloids". Pharmacological Research. 1995, 31(2), 103–107. ISSN 1043-6618
34. PAYO, A.; DOMINICIS, M.; MAYOR, J.; OQUENDO, M.; SARDUY, R. "Tamizaje fitoquímico preliminar de especies del género Croton L". Revista Cubana de Farmacia 2001, 35(3), 203–206. ISSN 1561-2988
35. LIU, D; SHENG, J; LI, Z. "Antioxidant activity of polysaccharide fractions extracted from Athyrium multidentatum (Doll.) Ching". International Journal of Biological Macromolecules. 2013, 56, 1– 5. ISSN 0141-8130
36. MURILLO, E.; LOMBO, O.; TIQUE, M.; MÉNDEZ, J. "Potencial antioxidante de Bauhinia Kalbreyeri Harms (Fabaceae)". Información Tecnológica. 2007, 18(6), 65–74. ISSN 0718-0764
37. PRIOR, R.; WU, X.; SCHAICH, K. "Standardized Methods for the determination of antioxidants capacity and phenolics in foods and dietary supplements". Journal of Agricultural and Food Chemistry. 2005, 53(10), 4290–4302. ISSN 0021-8561.
Recibido: 17/10/2017
Aceptado: 11/01/2018
MSc. Clara Azalea Berenguer-Rivas, Pharmacy Department, Universidad de Oriente, Cuba, clarabr@uo.edu.cu