INTRODUCTION
Resistance to antibiotics is a serious problem that affects all countries, and precisely one of the key factors for the increase in antimicrobial resistance rates is the reckless use of antibiotics.1 In this case, the respiratory tract infections have a high epidemic potential by their easy transmission, especially those caused by antibiotic-resistant Gram-positive and Gram-negative bacteria like Klebsiella pneumonia, Mycoplasma pneumoniae and Haemophilus influenzae, multidrug-resistant Mycobacterium tuberculosis (Mtb), and multiazole-resistant fungi.2 Aditionally, they are responsible for one third of the mortality associated with infectious diseases, which represent 4.3 million deaths per year.3 For this reason, the development of new antimicrobial agents effective against these infections is required.
In this situation, the antimicrobial peptides (AMPs) also known as host defense peptides (PDHs) have a potent and specific antimicrobial action and therapeutic potential, so they constitute a promising alternative. They are a low molecular weight molecules produced by almost all existing life forms. Usually AMPs present between 10-100 amino acids, especially Arg residues and so most are cationic. In general, they are also are hydrophobic and amphipathic at physiological pH.4
The AMPs present a broad spectrum of activity against several pathogens both directly and indirectly (immunomodulation), which could be conveniently handled in order to direct and/or enhance the immune response whether or not in the presence of classical antibiotics. For this reason, they could be a suitable complement for anti-infectious therapy.4 AMPs show a wide spectrum of activities for a variety of microorganisms including fungi, bacteria, virus and parasites with a relatively less in vitro potency regarding current antibiotics.5
In mammals the AMPs have been identified in different secretions, tissues and cell types, including saliva, tears, sweat and milk, and in many different cell types, including epithelial/mucosal cells, macrophages, neutrophils, natural killer cells, monocytes, eosinophil leukocytes, Paneth cells, T and B cells.6 They are (i) constitutively synthetized in granules of the phagocytic cells (defensins in neutrophils and the paneth cells) or (ii) inducible (defensins in CD8T cells and in epithelial cells) or (iii) both constitutively and inducible (cathelicidins and defensins in a variety of cells, including neutrophils, epithelial cells, and macrophages) (table 1).7
AMPs play a fundamental role in respiratory tract defense for both the direct antimicrobial action and for playing as effector molecules accompanying the innate immune response. In this context there exists plenty of evidence that these peptides not only protect as endogenous antibiotics, but also with a range of alternative but not less important functions, including immunomodulation, inflammation and wound repair.8
Table 1 Innate defense molecules in the respiratoy tract9
| Antimicrobial peptides in the respiratory tract | Produced by cell |
|---|---|
| α-Defensins (HNP1-4) | Neutrophils |
| α-Defensins (HNP5) | Epithelial |
| β α-Defensins (HBD1-4) | Epithelial, Macrophages, monocytes, dendritic |
| LL-37 | Epithelial, Neutrophils |
| Lysozyme | Epithelial, Neutrophils |
| Lactoferrin | Epithelial, Neutrophils |
| Anionic peptides | Epithelial |
From the point of view of their application, AMPs have several advantages, since they rapidly eliminate pathogenic microorganisms5 and exhibit synergism with conventional antibiotics, lysozyme and many other molecules.10 Unlike antibiotics for clinical use that can indirectly cause sepsis due to the release of endotoxin from dead microorganisms, AMPs bind endotoxins and reduce the likelihood of septic shock.11 In addition, their pharmacological properties can be improved in several ways, since they are relatively easy to modify. Some of the strategies most used for the optimization of AMPs include cyclization, conjugation with nanoparticles, introduction of D amino acids, lipidation, encapsulation in liposoms, among others.12
GENERAL FEATURES OF AMPS PRESENT IN THE RESPIRATORY TRACT
In the respiratory tract, most abundant AMPs are lysozyme and lactoferrin but defensins, histatins, cathelicidins and small hydrophilic anionic peptides are also found.4
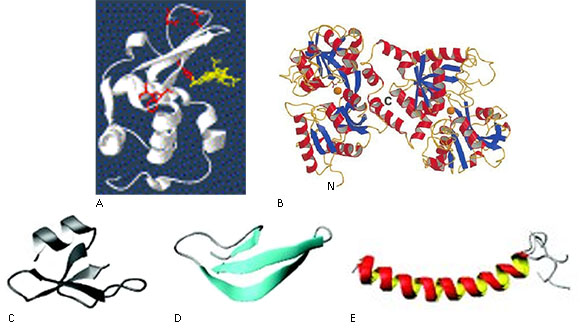
Lysozyme also called muramidase is an enzyme globular of 14.4 kDa that catalyzes the hydrolysis of β 1→4 bounds between acetyl glucosamine and N-acetylmuramic acid residues disturbing the formation of peptidoglycan, a major component of Gram-positive bacterium cell wall. Four disulfide bonds stabilize the molecule thereby presenting two domains: one α-domain with four α-helixes and one β-domain having a β-barrel structure (Fig. 1, A). It is produced by polymorphonuclear leukocytes (PMNs or neutrophils), monocytes, macrophages and epithelial cells.13
Lactoferrin is a monomeric glycoprotein of 80 kDa with intramolecular disulfide bridges, doubled in two known regions as N- and C-terminal lobes wich, in turn, are organized into two domains: N1 and N2, C1 and C2 belonging to the transferrin family that shows affinity for the metal iron (Fig. 1, B). Lactoferrin is particularly abundant in neutrophil granules and in the epithelial secretions. This molecule exerts its antimicrobial action by sequestering iron required for microbial respiration. In addition, it shows direct microbicidal activity located in the N-terminal fragment known as lactoferricine. Another fragment with antimicrobial activity is kaliocin-1,14 which is a cysteine-stabilized peptide of 31 amino acid residues what includes the sequences from positions 153 to 183 of human lactoferrin.9
Human defensins are a family of cysteine-reach peptides ranging about 3-5 kDa. They contain six cysteine residues that form disulfide bridges characteristic for this family. In the respiratory tract it is possible to find both β- defensins (Fig. 1, C) and α-defensins (Fig. 1, D). The later includes six human peptides from neutrophils (HNPs) and two from an epithelial origin (HDs),15) whereas the first ones summarize six members (hBD1-6).8
Histatins are members of a family of linear cationic peptides secreted by salivary glands in ranges from 50 to 425 µg/mL in healthy adults. They encompass 12 members from wich only three appear in natural form in saliva (Histatin-1, -3 and -5) whereas the rest are proteolytic products of them. They are low molecular weight proteins and contain 7- 38 amino acid residues from wich at least 12 are histidins. Their action is mainly against Candida sp., although gram-positive bacterium growth and other periodontal pathogens as Porphyromonas gingivalis can be inhibited by histatins. They are only found in oral cavity, which can be placed as a first defense line for infections of respiratory tract.16
Cathelicidins comprise a big family of AMPs containing a conserved N- terminal and heterogeneous C-terminal domains. The LL-37 peptide is relevant within the family, an AMP showing a potent activity against gram-negative bacteria.14 It appears inside neutrophils but can be secreted by epithelial and mast cells in different sites.15 LL-37 (Fig. 1, E) is the only cathelicidin that is expressed in humans and it is produced by proteolytic processing of its precursor hCAP18 (human cationic antimicrobial protein).15 It comprises 37 amino acid residues and posseses the ability to neutralize bacterial lipopolysaccharide (LPS). In addition it can also act directly on the DNA. LL-37 also exerts chemotaxis on neutrophils, eosinophils, T- cells and mast cells, modulates dendritic cells, induces chemokine synthesis in epithelial and monocytes and can induce angiogenesis and in vitro epithelial wound healing.8
Small hydrophilic anionic peptides (AAMPs) containing homopolimeric regions (5-7 aspartic acid residues) and net charge between -1 and -7, present post-translational modifications which are critical for their activity. Their mechanism of action is based in the interaction with the microbial plasma membrane for which they adopt amphiphilic structures. They were first isolated during the 80 s and since then they have been considered an important part of the innate immune system. They have been found in lungs, broncho-alveolar washes and in the lung surfactant substance. They show a wide spectrum of action against bacteria, fungi and viruses although in some cases their antimicrobial activity appear as a secondary function both as constitutive or induced molecules. On the other hand they require the presence of Zn-ions as cofactor which can change their negative charge while interacting with membranes. One of the AAMPs reported as important for the respiratory tract is SP-BN, a derivative of the N-terminus of the surfactant protein SP-B with aproximatelly 80 amino acid residues which is secreted together with further surfactant components.17
AMPS AND MULTIRESISTANT RESPIRATORY INFECTIONS
Respiratory infections can be caused by a variety of microorganisms that include bacteria, viruses, fungi and even parasites; since the airways are constantly exposed to particles, vapors, aerosols and microbial pathogens.21
Regarding bacterial infections, those caused by K. pneumonia stand out because it is one of the multi-drug resistant (MDR) organisms considered an urgent threat to human health by the World Health Organization (WHO).22 In addition, it is among the bacteria that can most easily develop mechanisms of resistance to multiple classes of antibiotics, and in fact, the rates of drug resistance have increased at an alarming rate.23
In this scenario, different AMPs have been evaluated and promising results have been obtained. One of the AMPs with activity against multi-drug resistant clinical isolates (MDR) of K. pneumoniae is S-thanatin, an analogue of thanatin (a cationic antimicrobial peptide isolated from the insect hemiptera Podisus maculiventris). It showed in vitro a minimum inhibitory concentration (MIC) in the range of 4-8 μg/mL. When S-thanatin was applied in in vivo studies, using a model of mice infected with this bacterium, the survival rate was increased, the bacterial colony forming units (CFU) of the intra-abdominal fluid and the level of LPS in the plasma were reduced.24 It has been described that this AMP acts on the phospholipids of the cytoplasmic membrane destabilizing it and this results in the release of periplasmic material and cell death. In addition, it was determined that it acts on the respiratory chain.25
In another study, a cathelicidin antimicrobial peptide was identified from Alligator mississippiensis (American alligator) and it was demonstrated that cathelicidin has a strong activity against different MDR bacteria such as K. pneumoniae.26 Another AMP with activity against K. pneumoniae is Cm38, which was purified from the scorpion venom Centruroides margaritatus, and showed a MIC of 64 Μm.27 On the other hand, the synergistic effect of the antimicrobial peptide LL-37 with azithromycin at submicromolar doses against K. pneumoniae has also been described.28
Another important bacterial infection is the tuberculosis (TB) caused by M. tuberculosis, which is together with HIV / AIDS one of the most lethal infectious diseases worldwide. In addition, currently few effective therapeutic options are available, since in the last 40 years only the novel antituberculosis drug bedaquiline, approved in 2012, has been commercialized. For this reason, a large part of the scientific community has focused on the use of AMPs as anti-tuberculosis drugs. One of the important advantage of these molecules for the treatment of TB is that they can be used in different ways: (i) stimulation of endogenous AMP production for direct bacterial elimination, (ii) administration of AMP also for direct destruction, and (iii) modulation of the innate immune response by endogenous and externally applied AMP.29
In recent years, many AMPs (table 2), including synthetic compounds and all those that are expressed in lungs, have been evaluated against mycobacteria. Among them are the defensins hNP-1, hNP-2 and hNP-3, which have been shown to be active against M. avium-M. intracellulare. In particular, hNP-1 is active against ten different strains at 50 μg / ml. It was also active against tuberculosis in mice infected with 1.5 × 104 CFU of Mtb H37 Rv.30
Another of the AMPs evaluated against Mtb is LL-37 wich represents one of principal humoral tools in the defensive cascade. Here the presence of the bacterial compounds represent strong stimuli for its secretion in different anatomical locations as tongue, oral and pulmonary mucosa. LL-37 not only can exert a direct antibacterial action but also can immunomodulate, induce cell migration, proliferation and apoptosis of infected cells and has a significant influence in the inflammation process. Its antibacterial activity is especially relevant in the infection with M. tuberculosis in the early phase of infection. In the late phase, a substantial contribution for macrophage recruitment occurs with the help of human neutrophil α-defensins.31
Additionally, the lactoferrin has been effective in to block intracellular growth in vivo of M. tuberculosis.14 Several derivatives of lactoferrin from human and bovin origin have also been evaluated of human and bovine origin against M. avium, which showed antimycobacterial activity with LD50 values between 10and 40 Μm.32
In this regard, research has also been conducted to determine the synergistic or antagonistic effect of AMPs with classical antimycobacterial drugs. For example, the combination of hNP-1 with isoniazid and / or rifampin showed synergistic effects in vitro, ex vivo and in vivo.33 On the other hand, the combination of hBD-1 with isoniazid reduced the growth of Mtb.34
The mechanism of action of AMPs to fight infections caused by M. tuberculosis has not yet been described but Sonawane et al give a possible explanation. These researchers suggest that in the latent phase of mycobacterial infection, the membrane of the host cell is altered, which causes the exposure of negatively charged molecules and thus favors the union of the AMPs. However, in the acute phase the bacilli have overcome the immune system and begin to multiply. In this case, AMPs can attack mycobacteria directly.35
However, although they are less frequent, fungal infections are also an important cause of respiratory infections. They can be found mainly in immune compromised patients but also in healthy persons visiting contaminated caves (Histoplasma capsulatum) or exposed to asbestos sprays or pigeon dejections (Aspergillus fumigatus) causing bronchopulmonary allergic aspergilosis, aspergiloma and invasive pulmonary aspergilosis. In addition, Scedosporium apiospermum and Lomentospora prolificans (previously Scedosporium prolificans) are "emerging" human pathogens that cause mainly pulmonary infections.36 For immune compromised patients mycosis in respiratory pathways is opportunistic and caused by Candida sp., Criptococcus neoformans, H. capsulatum, Aspergillus sp. and Pneumocystis jirovecii.37 Despite treatment, most invasive fungal infections are associated with high mortality rates of > 50 %.3
Candida sp. is known to colonize the oral cavity of immunocompromised patients for which AMPs as histatins are efficiently. Particularly histatin 5 is the most antifungal tool. It mechanism of action is based in the bound of the peptide tom proteins present in the cell wall of C. albicans like Ssa1p and Ssa2p.38 Later the internalization of the salivary gland-secreted cationic histatin, a process dependent of the presence of polyamine transporters Dur3 y Dur31,39 provokes the loss of mitochondrial membrane integrity with the consequent efflux of ATP and K+ ions trough the exterior of the yeast.40
Pneumonia produced by P. jirovecii is the most common infection in persons with human immunodeficiency virus (HIV). The immune response against this pathogen includes both innate and adaptive mechanisms. In vitro studies demonstrated that human lactoferrin suppressed the growth of this fungus in more than 50 % at 20 mg/L.41
Aspergillus fumigatus causes invasive aspergillosis in immunocompromised individuals with high mortality rates, which is an important clinical concern. At present many of the antifungal agents available for the treatment of this infection are ineffective, however, many AMPs have shown encouraging results. Some of them are a family of 6 lipidated antifungal AMPs composed of 4 amino acids joined to aliphatic chains of different length showing activity against A. fumigatus. Particularly, one of them had better activity than amphotericin B and in vitro and in vivo increased the life rate and survival of mice infected with A. fumigatus. According to studies carried out, these AMPs have an effect on the detergent-type plasma membrane.42
On the other hand, viruses frequently cause infections in the respiratory tract. The most common are influenza, adenoviruses, parainfluenza, rhinoviruses and human respiratory syncytial virus (RSV). The mechanisms of action for an efficient (or not) defense include humoral tools as antibodies and AMPs that could act in a synergic way with other actors of the immune response. For antiviral AMPs, three main mechanisms of action have been described: (i) inhibition of the binding of the virus to the membrane of the host cell; (ii) alteration of the viral envelope; and (iii) inhibition of virus replication by interaction with the viral polymerase or transcriptase.43
Among AMPs with a remarkable antiviral activity we can find HNPs, HDs y hBD2 active against influenza mainly but also against adenovirus and parainfluenza. They also contribute to the defense controlling the infections produced by rinovirus inducing the production of cytokines and recruiting dendritic cells and T lymphocytes. In addition, lactoferrin can prevent infection of new cells by viruses with both DNA and RNA genomes by direct binding of the virus and blocking host receptors used by viruses to gain entry into cells.44
One of the most common respiratory infections of viral origin is caused by the virus influenza specifically of the type A. This virus is a major cause of morbidity and mortality in the United States and it is estimated that at least 31 000annual deaths in the United States are related to the same. Currently there are vaccines against this virus; however, new antiviral drugs would favor the treatment of these infections. Among these possible new antiviral drugs are AMPs (table 3), including a peptide derived from the signal sequence of the factor 4 of fibroblast growth (EB, entry blocker). This peptide showed broad-spectrum antiviral activity against influenza viruses, including subtype H5N1 in vitro, and was protective
in vivo. Regarding its mechanism of action, it was reported that it inhibits the binding of the virus to the cellular receptor.45
Another of the peptides studied and that has presented activity against influenza viruses is lactoferrin. This peptide can interact with the virus and also with its receptors on the cell membrane of the host cells. Taking into account these properties of lactoferrin researchers synthesized three new peptides lactoferrin analogues (SKHSSLDCVLRP, AGDDQGLDKCVPNSKEK and NGESSADWAKN), which inhibited the influenza virus (H1H1, H3N2) to femtomolar concentrations.43
RSV accounts forapproximately 20 % of all lower respiratory infections in infants, but it can also cause deadly infections in immunocompromised individuals. In addition, so far there is no vaccine to combat this viral infection but many investigations are dedicated to the search for effective antiviral agents against RSV. In a study it was shown that human LL-37 cathelicidin has antiviral activity against RSV in vitro, which is due to a central portion of the peptide. In addition, it significantly inhibited the synthesis of new viral particles.46 Other researchers evaluated the action of a peptide derived from the RhoA protein 80-94, which inhibited several strains of RSV at submicromolar concentrations in vitro. On the other hand, Pastey et al. demonstrated that another peptide also derived from RhoA 77-95 inhibited the replication of RSV in the lungs ofmice when administered intranasally before, or shortly after, the viral challenge.47
In recent years, parasitic diseases affecting the respiratory system, mainly the lungs, have become of increasing important because of the incidence in the immune compromised population. The most common diseases are pneumonias caused by Plasmodium sp., Entamoeba histolytica, some species of the genus Leishmania, Trypanosoma sp., Toxoplasma gondii, Ascaris lumbricoides, Strongyloides stercoralis, Ancylostoma duodonale, among others. Once inside of the body, these parasites travel to the lungs, most often through the blood. Migration of the parasite in adult form causes obstruction of the airways whereas the larva causes inflammation. In response to these parasites, an increase occurs in IgE and IgG antibody levels.48 Another innate mechanism is constituted by the action of the peptide LL-37, which has shown to be direct and indirect activity against many parasites as Leishmania sp. and Entamoeba histolytica.49
Conclusions
The antimicrobial peptide concept has recently expanded to the wider term: “host defense peptides” because not only a direct killing action has been demonstrated for them but also a relevant immunomodulation activity has been observed, studied and handled. Taking in account that classic antibiotics, as microbial secondary metabolites present no functional relation with the Immune System, AMPs are emerging to a remarkable group of humoral effectors that are being considered, with all property, as the antibiotics of the future. Its incidence in respiratory and pulmonary diseases is relevant and therapeutic interventions using these peptide molecules from both human and animal sources could become a part of current medical practice in the near future