Introduction
Radiopharmaceuticals are medicines that deliver a predefined amount of radiation to a target tissue for diagnostic or therapeutic purposes depending on the mechanism of decay. Radiopharmaceuticals are usually made of two parts: a “radioactive core” and a “carrier system”; the latter allows the irradiation of malignant cell populations, avoiding damage to healthy tissues [1].
Beta-emitting radionuclides are usually produced mainly by direct reaction in dedicated targets using neutrons from nuclear reactors. By means of those reactions it is possible to produce a large number of isotopes and different nuclei in the target. The chemical methods to extract the desired radionuclide leads to the presence of a considerable amount of carrier. In this case, the specific activity, which is the ratio between the activity of the radioisotope and the mass of the element taken into account, is very low.
High specific activity is essential both for therapeutic and for diagnostic radiopharmaceuticals, especially in the case of radioimmunotherapy (RIT) and peptide receptor radionuclide therapy (PRRT), since cancerous cells have only a few selective sites. or diagnostic uses, high specific activity allows the use of a very small amount of radiotracer, which helps to maintain the physiological aspect of the process as much as possible [2].
The accelerators based on the ISOL (Isotope Separation On-Line) technique might be an efficient way to produce radionuclides for radiopharmaceuticals application, thanks to the mass separation, which guarantees the possibility to produce radiopharmaceuticals with large specific activity, close to theoretical value. Today, the ISOL technique is established as one of the main techniques for the on-line production of radioactive ion beams [3].
At INFN-LNL (Istituto Nazionale di Fisica Nucleare - Laboratori Nazionali di Legnaro), the SPES (Selective Production of Exotic Species) facility will allow the production of radioactive ion beams of neutron-rich nuclei with high purity, in the range of mass between 80 and 160 amu [4].
The ground-breaking idea of the ISOLPHARM method was granted an International patent (INFN). The driving idea is to obtain carrier-free radiopharmaceuticals thanks to the extreme purity of ISOL radioactive beams.
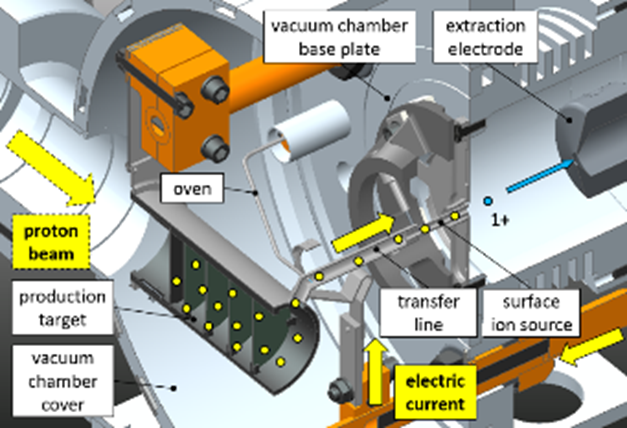
At SPES the production of the radioactive isotopes is obtained by nuclear reactions induced by 40 MeV protons, accelerated by a cyclotron, recently installed at LNL, that will collide a multi-foil target with discs of uranium carbide [5], properly spaced in order to dissipate the heat (8 kW) generated by the reaction. The produced radioactive isotopes will have atomic numbers between 28 and 57. Most of the produced nuclides will be neutron-rich, as in the chart of nuclides. The reaction products will be extracted from the target by evaporation at high temperature (about 2000°C), and then forced to pass through a transfer tube towards an ionization cavity, where they will be ionized to the 1+ state [6]. Once ionized, these isotopes will be accelerated through an electrode at high potential (up to 40 kV).

Figure 1 Chart of the nuclides produced from 238U fission target after irradiation with protons at the current of 200 μA and energy of 40 MeV.
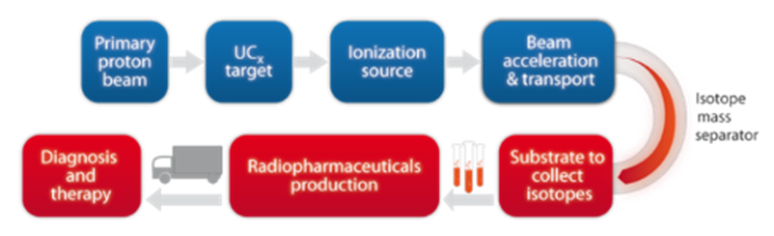
The formed beam will be focused using different electromagnetic systems and purified in order to have a pure isotope beam without any contaminants. It will therefore be possible to collect the radionuclides of interest using a proper substrate placed at the end of the experimental line. In figure 3 a general scheme of the process is shown. When the mass separation is performed effectively (ΔM/M at least than 1/200) only an isobar chain is present on the collection target. The radionuclide quality is therefore expected to be extremely high. Therefore, the ISOL technique is now under study for the production of radionuclides for nuclear medicine.
The radioisotopes produced using uranium carbide that are interesting from a radiopharmaceutical point of view are: 89Sr, 90Y, 125I, 131I and 133Xe. Feasibility studies using stable ion beams for the production of strontium, yttrium and iodine are described in this work.
The ISOLPHARM project as a first step aims to the production of radionuclides already present on the market. Nevertheless, this can be regarded as innovative because of the possibility of producing them as carrier-free radionuclides. As a second step, the ISOLPHARM project suggests the study, as a research facility, of innovative radionuclides coming from a fissile target bombarded with high currents and energies.
Materials and methods
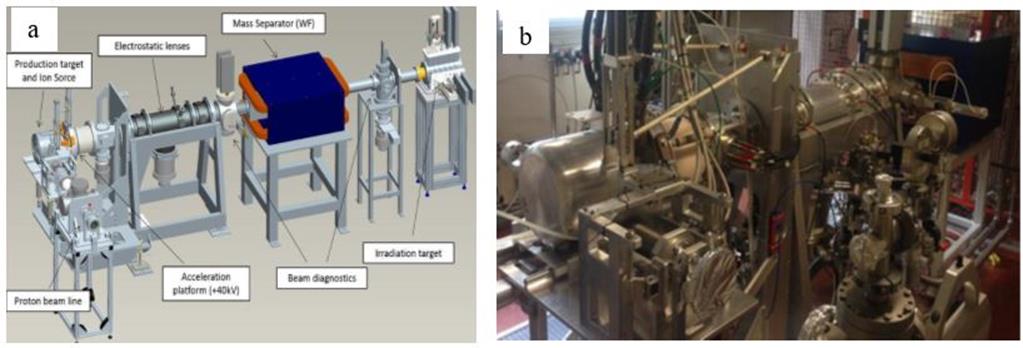
The experimental apparatus present at LNL allowed the performance of some preliminary tests. A SPES test bench was used, referred to as Front End offline (FE). This apparatus, shown in figure 4, has been designed and developed for the SPES project.
To study the production of a radionuclide with the ISOL technique to obtain a radiopharmaceutical product, stable isotopes of the same element can be used, since they have the same chemical behavior. For this reason, the FE was used to produce stable ion beams and carry out the feasibility tests here reported.
The FE is made of five different functional subsystems: the ion source complex, the beam optics subsystem, the Wien filter, and the diagnostic boxes 1 and 2. The ion source complex is placed inside a vacuum chamber that can guarantee the use of the ion source at high temperatures with pressure levels between 10-5 and 10-6 mbar. In offline modality, different methodologies can be used to introduce the stable isotopes to be ionized and accelerated, depending on the physical state of the element. In the case of gases, they are introduced through a controlled gas flow and injected in the ionization source thanks to a calibrated leak; in case of solid materials, they are in the form of soluble salts, dissolved in acidic media and quantitatively deposed and solvent evaporated on a tantalum foil, called mass marker (MM). The mass marker is then carefully folded and inserted into a heated tantalum tube, called oven, that allows the element atomization and injection into the ionization source. The ionization sources used in the experiments later described are of two kinds, according to the first ionization potential of the element. For elements of the 1st and 2nd groups, a Surface Ion Source (SIS) is adopted; for elements with higher electronegativity, a Plasma Ion Source (PIS) is necessary.
Between the ion source and the extraction electrode an acceleration voltage up to 40 kV is set. The beam optics subsystem is made of a set of electrostatic steerers and a quadrupole triplet that allow respectively the beam alignment and focalization. In diagnostic box 1 there is a Faraday cup for beam intensity monitoring and a grid-based beam profile detector. The Wien filter is an electromagnetic mass separator used in the FE to generate a pure isobaric beam. Following mass separation, a second diagnostic box is installed, constituted by a Faraday cup, a grid-based beam profile detector and an emittance meter device.
For the simulation of the radionuclide production, at the end of the line, immediately after the emittance meter, a substrate of pharmaceutical grade is positioned in order to collect the desired accelerated stable ions. Different substrates were designed and produced, the materials were also chosen considering the need to make, as easiest and fastest as possible, the transformation of the substrate into a radiopharmaceutical. Among the biocompatible materials, sodium chloride was chosen for the deposition of two of the tested elements: strontium and yttrium. For iodine, instead, activated carbon was the material of choice. In all cases the powders, alone or blended with ligands, were compressed to obtain compact disks with a diameter of 40 mm.
Sodium chloride disks were obtained by pressing different amounts of sieved (300 μm) NaCl powder in an industrial press. The mold die allowed obtaining disks of 40 mm of diameter with the application of a strength of 30 ton for 5 minutes; no ligand was used. For the deposition of strontium, the disks were produced pressing sodium chloride TraceSELECT (Fluka Analytical) to avoid traces of strontium present as contaminants in the others. For yttrium deposition, instead, sodium chloride (Sigma Aldrich) was used, since no yttrium traces are present.
For iodine deposition Activated Carbon was chosen as the secondary target material. Sodium chloride could not be used because it has, as an intrinsic contaminant, iodine in the anion forms of iodate and iodide. Moreover, iodine is in the gaseous form at the operating pressure conditions (10-5-10-6 mbar) thus possibly causing iodine ions to be lost immediately after being implanted in the secondary target. Thus, activated carbon was chosen as an ideal material for matrix development, because it can efficaciously trap iodine [7]. Vinyl Alcohol (PVA) demonstrated to be a good ligand for activated carbon.
An analytical method has been established for each element in order to quantify the effective amount of atoms deposed on the substrate after beam irradiation; both the nature of the element and of the matrix have been taken into consideration.
Results and discussion
In the case of sodium chloride disks, after irradiation they were dissolved in a proper solvent for chemical quantitative analysis. For strontium, it was used Atomic Absorption Spectroscopy with atomization in a Graphite Furnace (GF-AAS), (GTA 110 with PSD 120 auto sampler, Varian) and the hollow cathode lamp for strontium (Heraeus), at an operating wavelength of 460 nm. A strong acidic matrix was used (HNO3 0.3 M) in order to reduce non-specific absorbance phenomena due to the presence of NaCl.
For yttrium a different analytical method was chosen since, due to its low volatility, it cannot be observed with AAS.23 ICP-OES (Inductive Coupled Plasma-Optic Emission Spectroscopy).
In the case of iodine quantification, a process was developed to extract iodine deposited in the carbon matrix. 10 ml of a 0.5 M solution of NaOH were mixed with the irradiated substrate. This solution was then treated with 1 M sulfuric acid in order to obtain an acidic pH. The solution was then automatically titrated (848 Titrino Plus - Metrohm) with sodium thiosulfate 0.001 M, after pretreatments with bromine water, formic acid and an excess of potassium iodide. Titration proved to be accurate and sensitive enough for iodine quantification.
Using the aforementioned apparatus and techniques, strontium, yttrium and iodine beams were produced and collected on the described substrates. In table 1 the main experimental conditions are listed for each element.
Table 1 Main experimental conditions for the ionization and deposition experiments carried out for the three elements under study.
| Strontium | Yttrium | Iodine | |
|---|---|---|---|
| Surface Ion Source | Plasma Ion Source | Plasma Ion Source | |
| Strontium nitrate | Yttrium chloride | Potassium iodide | |
| Oven | Transfer line | Oven | |
| Sodium chloride, 3.6 g | Sodium chloride, 1.8 g |
Act. carbon 50% (w/w) PVA 50% (w/w), 1.3 g |
|
| GF-AAS (λ=460.70 nm) | ICP-OES (λ=371.03 nm) | Titration |
Before carrying out the depositions, ionization efficiency and focalization tests were performed. Ionization efficiency tests were carried out by evaporating the material contained in the mass marker and collecting the beam in the second Faraday Cup. By integrating the curve of current in time the number of ionized atoms can be obtained; this quantity can be compared to the number of atoms introduced in the MM to obtain ionization efficiency. To check qualitatively the accelerated ions, several mass scans using the Wien Filter were performed. The emittance meter was used to control beam dimensions, position and divergence for each element and to adequately set steerers and quadrupoles voltage.
In the cases of strontium and yttrium, during the deposition experiments, the beam current was periodically checked by introducing the secondary Faraday Cup for a few seconds. In the case of iodine, the substrate itself (a conductive material) was used as a third Faraday Cup, allowing to monitor the iodine current throughout all the deposition.
Conclusions
From this study the possibility to use Radioactive Ion Beams (RIBs) to obtain high purity radionuclides was demonstrated, since in all the cases the deposition of stable ion beams was efficient and the recovery quantitative. The chemical purification process for the elimination of isobaric contaminants is now under development. This technique opens the possibility to produce a wide range of radionuclides with extremely high levels of purity both in terms of specific activity, because of the lack of isotopic contaminants, and radionuclidic and chemical purity, since impurities coming from the beam and from the targets are very limited, compared to those of traditional methods.