Introduction
Myelomeningocele (MMC), which accounts for approximately 86.8 % of spina bifida cases, is the most common form of spinal dysraphism. They can be open or closed.1
The actual prevalence of myelomeningocele varies geographically. In South Africa, the incidence is 0.77 to 6.1 per 1000 live births and it is postulated that the incidence is even higher in rural areas.2) In the central belt of Nigeria, the reported incidence is up to 7 per 1,000 deliveries.3
From the physiopathogenic point of view, a neurulation failure occurs. This results in a disc-like opening, with the neural placode with the central canal as the dorsal feature, the anterior horn motor cells near the midline, and the sensory dorsal root entry zone on the lateral edges of the disk. The vertebrae, muscles, and skin (in the case of open MMC) do not fully form, and the resulting exposure of the central nervous system (CNS) to amniotic fluid, and mechanical contact thereof within the uterine environment, can lead to neural injury. With the failure of the spinal cord to form a tubular structure, there is also abnormal positioning of the spinal nerve roots.4
The primary treatment of open MMC is early surgical closure (less than 48 hours), to avoid infections of the nervous system from contact with body contaminants (such as faecal matter) or the environment, and to protect the neural structures. Closure involves invagination of the placode, disaggregation of nerve roots, and may include sectioning of the filum terminale. Delayed closure (more than 48 hours) is associated with a higher incidence of meningitis.1
Hydrocephalus, which accompanies MMC in up to 85 % of cases, can complicate the neurological picture.1,5
When access to surgical treatment is not available, which is common in low-resource settings, these children can survive, allowing the placode to epithelialize, which gives surgical repair distinctive characteristics.6,7 The management of these cases does not show clear guidelines.7) Therefore, the objective of this work is to characterize an initial series of open MMC cases that received late repair at the Hôpital de Référence de Maradi.
Methods
A series of five cases is presented, with open MMC, who received late surgical repair at the Hôpital de Référence de Maradi, Niger, between June - December 2022.
The data collected were: sex, age, size of the defect and its location, pre and postoperative neurological function, and hemoglobin values at the time of surgery. In addition, complications during the follow-up period were recorded.
The information was obtained from medical records and imaging records. The data was obtained with the prior informed consent of one or both parents, and approval by the Ethics Committee of the institution. The ethical principles for this study were complied with in accordance with the provisions of the Declaration of Helsinki.
The technique described by Watson et al.7) First, standard asepsis is performed, since the epithelialized surface does not present special problems of iodine intoxication. For patients with open MMC, iodinated agents are avoided because of the theoretical risk of iodine-induced hypothyroidism as well as potential damage to neural tissue.
An incision is made beginning at the midline of normal skin, one centimeter above the sac, and extending to the junction of normal and epithelialized arachnoid skin on either side of the sac. Circumferential incisions are then made to the sac, usually in its upper part, near the junction of the presumed placode and the epithelialized arachnoid. The technique requires that a plane be found between the skin and the placode. Begin by gently retracting the top of the skin ellipse, and identifying the neural plate, which will appear gray and friable, while the skin is firm and pale. This plane is dissected with a scalpel or sharp scissors until the entire ellipse of skin is freed from the placode.
Once the sac is opened, the placode is identified, although compared to its perinatal appearance, the margins of the epithelialized placode are less well defined and more like a keloid than exposed neural tissue. Nerve roots arising from the placode must be preserved, although root sacrifice, particularly those distal to the placode, can be done without further deficit. At this point, the residual skin is removed by sharp dissection.
Neurolization with suture was not performed, due to logistical conditions. Theoretically, microsurgical closure of the placode surface helps minimize scarring and will decrease the chance of reattachment, but this has not shown clinical repercussions.8 The inclusion of epithelium in deep closure should be avoided, as it is related to a higher incidence of epidermoid cysts in the future.9
The dural edges are then freed, meeting the epidural plane rostrally, and proceeding circumferentially with sharp scissors, placing one blade in the epidural space and the other in the sac. The epidural fat is the guide to stay in the correct plane, since the dorsal paraspinal fascia can be a false layer of dissection. The dura mater is then mobilized toward the spinal cord.
The dura is then closed tightly. The edges of the skin are mobilized. Facial relaxing incisions are generally not used. Prophylactic antibiotic therapy was used, with ceftriaxone 50 mg/kg of weight, for five days. The patient was discharged in 3-5 days if there were no complications.
Results
Five patients were intervened, three female and two male. In all cases the age of the patients at the time of surgery was greater than 30 days. Three cases showed a lumbosacral location of the defect, and the rest at the thoracolumbar junction. All the dysraphisms were open, showing signs of partial or total epithelialization of the placode, at the time of the intervention. During the evaluation of neurological function, three of them exhibited distal paraplegia, with reflex and sphincter involvement. Laboratory tests showed, in all cases, hemoglobin levels below 12 mg/dl, interpreting these values as a reflection of nutritional deficit. Only one case showed signs of hydrocephalus in the postoperative follow-up (case no. 5 table), due to a bulging fontanelle and progressive growth of the head circumference, which was treated with a ventriculoperitoneal shunt after 30 days. None of the cases showed deterioration of the neurological state with respect to the preoperative evaluation.
Table Clinical characteristics of the patients operated on for MMC
| Case | Age (months)/Sex | Defect Dimension (cm x cm) | lesion level | Preoperative neurological function | Pre-surgical Hb level (mg/dl) | Complications |
|---|---|---|---|---|---|---|
| 1 | 2/M | 7 x 4 | L3-S1 | No motor defect | 10,8 | - |
| 2 | 15/F | 10 x 5 | L2-S2 | Distal paraplegia, sphincter non-tonicity, psychomotor retardation, areflexia | 7,8 | - |
| 3 | 2/F | 9 x 4 | T11-S5 | Psychomotor retardation, distal paraparesis | 11,1 | - |
| 4 | 2/F | 10 x 4 | L1-L4 | Distal paraplegia, areflexia | 5,9 | - |
| 5 | 1/M | 12 x 6 | T10-L5 | Distal paraplegia, areflexia, no sphincter tone | 8,7 | VPD at 30 days due to communicating hydrocephalus |
First illustrative case
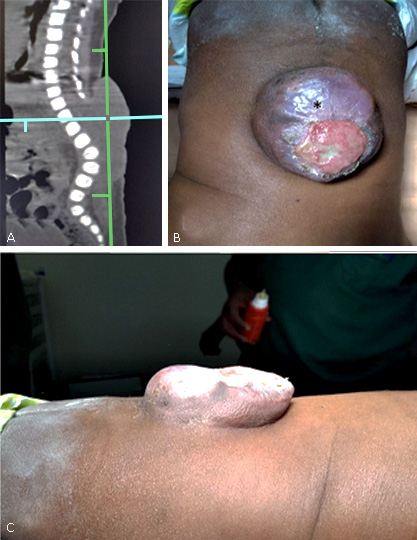
Two-month-old male patient. With a history of consanguinity (cousin marriage). Sicklemic mother, who did not receive prenatal consultation or nutritional contributions during pregnancy. Born by extra-hospital delivery. On physical examination, spinal dysraphism was observed at the lumbosacral level, with diameters of 7 by 4 cm, and complete epitalization, accompanied by foot varus. Bilateral Babinski response. No obvious motor defect to the nociceptive stimulus. After surgical repair with complete resection of the epithelialized surface, there was no femoral motor impairment. He is discharged on the fifth day after completing antibiotic therapy (fig. 1).
Second illustrative case
Female patient, two months old. No history of consanguinity or parental morbidities were collected. The mother did not receive prenatal consultation or nutritional supplements during pregnancy. She is brought in for consultation after two months of birth. On neurological examination, distal paraparesis to the nociceptive stimulus, weak extensor plantar response bilaterally. Upon inspection of the defect area, signs of partial epithelialization. Head circumference within normal parameters, no ventricular dilatation on transfontanellar ultrasound examination. He underwent surgery to repair the defect, with partial resection of the devitalized placode. At the post-surgical evaluation, without variation of the previous neurological state. After 72 hours of antibiotic treatment with Ceftriaxone 50 mg/kg of weight, he is discharged. He continues with Amoxicillin until completing the seven days. He had no complications during the 3-month follow-up period (fig. 2).
Third illustrative case
Female patient, two months old. No history of consanguinity. The mother received folate supplements in the last trimester. She is brought to the consultation after two months of birth, where extensive spinal dysraphism is found, apparently meningocele, with little neural material inside the sac, and signs of epithelialization at the lumbar level. On neurological evaluation, bilateral femoral motor defect to painful stimulus, without extensor plantar response (Babinski). Surgical intervention is carried out, where epitalized dural elements are resected, and vitalized neural plate is found inside the sac, which is dissected and preserved. He is discharged on the fifth day, after completing antimicrobial therapy. He has no complications during follow-up (fig. 3).
Discussion
Spinal dysraphisms are caused by defects in neurulation, an embryonic process involved in the formation of the neural axis and responsible for the formation of the brain and most of the spinal cord, up to approximately the junction of the S1 spinal cord segments and S2. It involves dorsal folding and midline fusion of the neural plate to form the primary neural tube. Because the two edges of the primitive neural plate are continuous with the embryonic cutaneous ectoderm, successful dorsal closure of the primary neural tube also ensures perfect fusion of the midline skin and mesodermal tissues.4,10
Complete failure of primary closure of the neural tube results in an open skin defect and an exposed, unfused neural plate, which is called an open neural tube defect.4
This incomplete closure generally leads to a malformation characterized clinically by neurogenic bladder and bowel dysfunction, as well as varying degrees of distal weakness.1
Optimally, the lesion should close within 48 hours of birth. This decreases the risk of infection of the nervous system, and possibly improves neurological function.11
In a retrospective cohort based on time of closure, children who underwent closure within 72 hours of birth have significantly better bladder status on urodynamic testing compared with children whose defects were closed later of 72 hours.12
Traditional MMC surgery aimed to preserve all nerve tissue for fear of loss of neurological function13 (usually negligible), even though the exposed placode was mostly devitalized and functionally disconnected from voluntary control.
This scheme is accompanied by two possible negative effects, which contribute to late post-surgical morbidity. First, meticulous preservation of all neural tissue often meant forcing a disproportionately large and bulky malformed neural placode into a tight dural enclosure recreated from scant meningeal remnants, leading to secondary symptomatic anchorage in even one third of the patients.14,15 Second, the fear of removal of “useful” neural tissue during dissection of the edges of the neural plate was often accompanied by the inadvertent inclusion of epidermal remnants within the repaired neural sac.16
The subsequent growth of these retained cells would explain the appearance of dermoid cysts.9 However, pathological studies of several resected placodes have found dermal tissue even within the plaque.16
Eibach et al.8) publishes a series of eight cases where he exposes a new paradigm focused on the aggressive but safe resection of the neural placode without loss of function, guided by intraoperative electrophysiology. The advantages include a greater ability of the cord to relate to the dural sac and, consequently, a lower risk of late tethering of the spinal cord together with a lower incidence of inclusion cyst. The obvious criticisms of this report are the small number of cases and the relatively short follow-up (average 15 months).
There are many surgical techniques described, mostly similar, based on the technique described by McLone et al. in 198013, who defended microsurgical closure, which involves approximation of the lateral edges of the open neural plate in the midline to reconstitute the neural tube. However, it is not clear whether this decreases the incidence of spinal cord tethering.17
On the other hand, the behavior of this entity in the African continent has its own characteristics, which make it difficult to follow these recommendations. Inadequate facilities, late presentation, shortage of trained personnel and microsurgical instruments, and absence of neonatal intensive care, identify the sub-Saharan panorama. Added to this is the poor nutritional status of patients, which makes them more prone to complications from surgery.18 The epitalization of the placode is a phenomenon typical of these environments, where repair is late, resulting from the healing of exposed neural tissue, which suffers repeated microtraumas and/or infection.7,19
We use the technique modified by Watson et al.7 This technique is suitable for late closure of large or epitalized myelomeningoceles, and does not require more extensive and complicated procedures. In this way, the sac is separated from the midline to avoid injury to the spinal cord near the neural placode. The MMC sac acts as a kind of tissue expander and the skin that covers the sac is used to cover the defect.
However, several procedures have been used to treat large MMCs, including Z flaps, skin grafts, latissimus dorsi flaps, fascial flaps, bipedal flaps, and tissue expansions 20, but these cases are the exception, not rule.
As with cases of primary closure, the size of the lesion and associated bone deformity must be carefully considered in judging the need for primary closure. Although the ability to do optimal closure is multifactorial and includes the size of the baby, the amount of viable skin, and the ability to correct bone deformity, especially kyphosis.20
Ozveren et al. classified myelomeningoceles in terms of the defect area as a percentage of the thoracolumbar area to allow selection of the surgical technique accordingly. They concluded that latissimus dorsi and gluteus maximus muscles combined with skin flaps have been shown to be safe for extensive MMC.21
However, these techniques require the cooperation of a plastic surgeon and are associated with increased operating time and blood loss. 11
In 10 % of infants, hydrocephalus will be evident at birth.1 In this group of patients with macrocranium and ventriculomegaly, simultaneous MMC repair and shunt placement may be appropriate. This approach is controversial and may increase the rate of complications. On the other hand, the sequential approach suggests that in neurologically stable infants with unchanged or slowly progressive ventricular diameter, close observation and evolutionary imaging studies can be followed (5) without repercussions on their subsequent neurocognitive status.22
While some authors have published that the frequency of infection and shunt dysfunction was similar with both regimens,23) Ozgural et al.24 found a lower rate of complications with the simultaneous scheme, however Gurbuz and Yuksel25) did not report any cases of infection using the sequential variant in their sample of 67 consecutive cases.
We follow a sequential approach, where the performance of the derivative procedure is based on the patient's own needs, during the clinical-imaging follow-up.
Although the usual treatment of babies with hydrocephalus and spina bifida is the placement of a shunt11, the implementation of the endoscopic third ventriculostomy with cauterization of the choroid plexus is a viable alternative, proposed as initial treatment in contexts with resources. limited.5 There is still no consensus on its use.
Conclusions
The surgical treatment of MMC in a low-resource setting, as is common in sub-Saharan Africa, has its own characteristics, marked by late presentation to specialized hospital centers, and the low nutritional status of patients. Surgical resection, partial or total, of an exposed and devitalized placode, with signs of epitalization, does not seem to influence the postoperative neurological status. There are no clear recommendations on the surgical management of this entity in the African context.