Introduction
Foot and mouth disease (FMD) is a highly contagious and economically devastating viral disease that affects cloven-hoofed animals, such as cattle, sheep, pigs, and goats. This disease causes significant morbidity and mortality, leading to substantial losses in production and trade.1) Foot and mouth disease virus (FMDV), a member of the Aphthovirus genus, exhibits remarkable evasive capability. FMDV, which exists in seven distinct serotypes and is characterized by rapid genomic variation, poses a significant challenge to control efforts. This adaptability stems from two key factors: error-prone RNA replication and extensive recombination. The RNA genome, which is susceptible to copying errors, introduces novel strains, while recombination further diversifies the viral landscape, potentially outsmarting existing immunity. Consequently, vaccines and diagnostic tools may become ineffective, paving the way for outbreaks and economic losses. Understanding the drivers of FMDV evolution is paramount for developing effective control strategies and safeguarding against future outbreaks.2,3) The first incursion of FMD in Egypt occurred in the 1950s with serotype O, establishing its lasting presence. Despite subsequent challenges from serotypes A (1967, 1972)4,5) and SAT2 (major outbreaks in 2012),6,7 O has maintained its dominance as the primary circulating strain across the country's governorates. A new lineage of serotype A FMDV has emerged in Egypt, posing a fresh challenge to livestock health and national biosecurity. This novel strain, FMDV-A-Egy-AHRI-RL385-Ven-2022, closely related to strains circulating in Venezuela and Colombia, suggesting potential pathways of transmission. Phylogenetic analysis revealed that the Egyptian isolates were closely related to the prototype strain A24 Cruzeiro and that their classification was within the topotype, EURO-SA. This lineage affiliation sheds light on the evolutionary history of the virus and its potential antigenic characteristics. Understanding the precise nature of FMDV-A-Egy-AHRI-RL385-Ven-2022 is crucial for developing effective control strategies and mitigating its impact. Further investigations into its virulence, transmissibility, and cross-protection with existing vaccines are essential for safeguarding Egypt's livestock industry and preventing further outbreaks.8 Control of the disease has been based on large-scale vaccinations with whole-virus inactivated vaccines, limitations of animal movements and destruction of herds exposed to the virus.9 The available vaccines generally show good protection against infection with homologous viruses and antigenically related isolates. Difficulties facing the eradication of FMD include the antigenic diversity of FMDV in nature, which has been reflected in the identification of seven serotypes (A, O, C, SAT1, SAT2, SAT3 and Asia1), 65 subtypes, until subtyping was interrupted, and multitudes of antigenic variants.10) In addition, many variant strains have been recognized within serotypes,11 and some of these differences may be important in relation to cross-protection. Therefore, serological tests are routinely used as part of the process for selecting the most appropriate vaccine strain for protection against a given field isolate.12 Nevertheless, vaccination may not provide optimal protection against sub serotypes and newly isolated field strains.6,13 In response to the emergence of a recent FMDV-A strain (FMDV-A-Egy-AHRI-RL385-Ven-2022), this study was aimed to comprehensively evaluate the cross-protective efficacy of currently available local and imported FMD vaccines. In response to the pressing need for reassessment, both locally produced and imported vaccines were undergoing urgent evaluation to address the risk of recurring FMDV outbreaks. Consequently, this study encompassed both in vitro and in vivo investigations, aiming to determine the efficacy of the existing vaccines in protecting calves against the recently isolated strain FMDV-A-Egy-AHRI-RL385-Ven-2022.
Materials and Methods
Virus
The Animal Health Research Institute isolated and identified FMDV-A-Egy-AHRI-RL385-Ven-2022 (A Venezuela) using real-time polymerase chain reaction (qPCR).8) This recently isolated and identified virus was then officially provided to the Central Laboratory for Evaluation of Veterinary Biologics (CLEVB) to assess the potency of the current inactivated FMDV vaccines, the provided viruses were tissue culture adapted for virus neutralization tests (VNTs) and virulent for challenge test. Additionally, FMDV A/EGY/1/2012 was obtained from the Strain Bank Department at CLEVB and was used as a homologous strain according to OIE guidelines.14
Cell line
The VNT was conducted using BHK-21 cells which were kindly provided by the FMD Department at the Veterinary Serum and Vaccine Research Institute (Abbasia, Cairo). These cells were specifically chosen for their suitability for FMDV studies and were cultured and maintained according to procedures described by OIE and Ferreira.14,15
FMD-inactivated vaccines
This study employed two inactivated FMDV vaccine batches (n=2) to investigate their cross-protective efficacy against the recently isolated FMDV-A Venezuela strain: a locally formulated trivalent oil-based vaccine (batch 1) containing the local isolates O/EGY/4/2012, A/EGY/1/2012 (A Iran-05 lineage), and SAT2/EGY/2/2012 and an imported polyvalent vaccine (batch 2) consisting of O Manisa, O-3039, A Iran-05, A Saudi-95, Asia-1 Shamir, and SAT-2. The selection of both vaccines was based on their prior satisfactory evaluations conducted by the CLEVB.
Calves and experimental design
A total of 26 native bred calves, aged 6-8 months and weighing approximately 200-300 kg, were provided for experimental purposes by CLEVB, divided into four groups and maintained under veterinary care in separate breeding stables with access to regular concentrated rations and water. Prior screening using VNT confirmed the absence of specific antibodies against FMDV (seronegativity). The specific roles of each group in the experiment, including vaccination and challenge details, are outlined below:
Group I (n=2): two calves were subjected to virus titration, specifically with the recently isolated FMDV-A-Egy-AHRI-RL385-Ven-2022 strain.
Group II (n=10): ten calves received subcutaneous vaccination with a previously evaluated local commercial FMD vaccine (batch 1). Five of these calves were then challenged with the homologous A/EGY/1/2012 strain, while the remaining five were challenged with the heterologous FMDV-A-Egy-AHRI-RL385-Ven-2022 strain.
Group III (n=10): ten calves received subcutaneous vaccination with a field dose of an imported FMD vaccine (batch 2) according to the manufacturer's instructions. Five calves were then challenged with the homologous A/EGY/1/2012 strain, while the remaining five were challenged with the heterologous FMDV-A-Egy-AHRI-RL385-Ven-2022 strain.
Group IV (n=4): four calves served as the nonvaccinated control group for the challenge test, ensuring the validity of the experimental results.
All groups were monitored daily for clinical lesions throughout the experiment. Samples were collected from clinically affected animals for further analysis.
Serum samples
The VNT was conducted to evaluate the humoral immune response in serum samples collected from vaccinated groups (II, III) and control group (IV) at days 0, 7, 14, 21, and 28 post vaccination. The assay measured antibody levels against both the vaccine strain and the FMDV-A-Egy-AHRI-RL385-Ven-2022 isolate.
Viral neutralization assay and calculation of the r1-value
Bovine sera collected from animals vaccinated in groups II and III were subjected to a two-dimensional microneutralization assay (MNT) to quantify the relative antigenic homology (r1-value) between the recently isolated FMDV-A-Egy-AHRI-RL385-Ven-2022 virus and the A/EGY/1/2012 (A Iran-05 lineage) vaccine strain. The MNT was performed following the established protocol described by Rweyemamu.16 The serological relationships (r1-values) were calculated and Interpretation according to OIE guidelines.14,17
/
The interpretation of the r1-value is as follows:
If r1-value < 0.3, it indicates a substantial level of antigenic variation from the vaccine strains. It is advisable to opt for an alternative vaccine strain, as the existing vaccine may not effectively address the observed antigenic differences.
If r1-value > 0.3, it signifies that there is significant similarity between the vaccine and field strains. This suggests that the vaccine is likely to offer effective protection against the field strains, as there is a notable level of antigenic resemblance.
Virus titration
Viral titration of the challenge test strain, FMDV-A-Egy-AHRI-RL385-Ven-2022, was conducted according to procedures described by Dekker, et al.18) The virus titer was subsequently calculated and expressed as log10 Bovine infective dose (BID50)/mL according to Karber.19
Challenge test
Twenty-eight days after vaccination, the challenge test was conducted using FMDV A Venezuela and FMDV A Iran-05 to vaccinated animals (groups II and III) and control animals (group IV) as described in the experimental design animals. The challenge test, calculation and interpretation were carried out according to OIE guidelines.14,20 The calves designated for the challenge underwent tranquilization both before and during the daily examination. Throughout a period of 7 days, the calves were diligently monitored for any notable clinical signs, particularly focusing on manifestations such as tongue and feet ulcers indicative of FMD. Infected animals received veterinary care and medical treatment until complete recovery and were then moved to a designated room for ex-experimental animals.
Ethical approval
The Institutional Animal Care and Use Committee at CLEVB reviewed the research manuscript and confirmed its compliance with bioethical standards and best practices. The CLEVB acknowledges the receipt and review of the research manuscript. Based on its assessment, the manuscript is deemed to uphold established bioethical standards.
Results
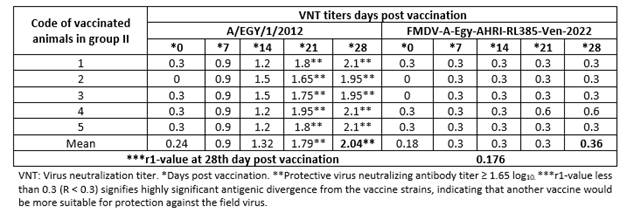
The infectivity titer of FMDV-A-Egy-AHRI-RL385-Ven-2022 indicated 106 BID50/0.1 mL. Weekly measurements of neutralizing antibody titers in sera from cattle vaccinated with the local inactivated FMDV vaccine (batch 1) revealed that they developed protective effects (≥ 1.65 log10)14) against the homologous virus A/EGY/1/2012 as early as 21 days post vaccination. However, despite testing until day 28, no detectable neutralizing antibody titers against the recently isolated FMDV-A-Egy-AHRI-RL385-Ven-2022 virus were observed. The highest antibody titers against A/EGY/1/2012 were recorded on day 28, as presented in Table 1. The r1-value for FMDV-A-Egy-AHRI-RL385-Ven-2022 was 0.176 with A/EGY/1/2012.
Table 1 Neutralizing antibody titers for homologous neutralization (A/EGY/1/2012) and heterologous neutralization of FMDV-A-Egy-AHRI-RL385-Ven-2022, in sera from cattle vaccinated with a local commercial trivalent inactivated FMDV vaccine batch (1).
Testing sera of cattle vaccinated with the imported inactivated FMDV vaccine (batch2) revealed a gradual increase in neutralizing antibody titers against the homologous A/EGY/1/2012 virus until day 28 post vaccination, as shown in Table 2, protective titers (≥ 1.65 log10) were achieved by day 21. However, no detectable neutralizing antibody response against the recently isolated FMDV-A-Egy-AHRI-RL385-Ven-2022 was observed throughout the 28-day period. Consistent with those vaccinated by FMDV vaccine (batch1), the highest antibody titers against A/EGY/1/2012 were recorded on day 28. The calculated r1-value for FMDV-A-Egy-AHRI-RL385-Ven-2022 was 0.175 with A/EGY/1/2012.
Table 2 Neutralizing antibody titers for homologous neutralization A/EGY/1/2012 and heterologous neutralization FMDV-A-Egy-AHRI-RL385-Ven-2022 for sera from cattle vaccinated with the imported inactivated FMD virus vaccine batch (2).
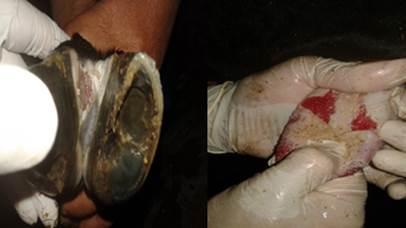
Cattle vaccinated with the local commercial inactivated FMDV vaccine (batch 1) were fully protected (100%) against the homologous A/EGY/1/2012 strain, as shown in Table 3. However, these strains displayed no detectable protection (0%) against the recently isolated FMDV-A-Egy-AHRI-RL385-Ven-2022 strain. Similarly, the cattle vaccinated with the imported inactivated FMDV vaccine (batch 2) achieved 100% protection against A/EGY/1/2012 but exhibited only limited protection (20%) against FMDV-A-Egy-AHRI-RL385-Ven-2022 (Table 4). The results of the challenge test were recorded after 7 days of virus inoculation by inspection of the tongue and both limbs of the inoculated groups of cattle to detect the characteristic lesions as shown in Figure 1.
Table 3 Detection of characteristic FMD lesions after homologous challenge (A/EGY/1/2012) or heterologous challenge with FMDV-A-Egy-AHRI-RL385-Ven-2022 in cattle vaccinated with a local commercial trivalent inactivated FMD virus vaccine (batch 1).
Table 4 Detection of characteristic FMD lesions after homologous challenge A/EGY/1/2012 and heterologous challenge with FMDV-A-Egy-AHRI-RL385-Ven-2022 in cattle vaccinated with an imported inactivated FMD virus vaccine (batch 2).
Discussion
The devastating impact of FMD on Egyptian livestock production has led to its classification as a sovereign and endemic disease. Despite government eradication efforts, FMD outbreaks persist due to the circulation of diverse serotypes and the emergence of new lineages within the SAT2, O, and A serotypes. Animal movements and international trade, particularly livestock imports from Sudan, India, and South America (Brazil and Colombia), as documented in a 2021 USDA report, are identified as key risk factors for the introduction of new FMDVs, including serotypes and lineages not included in current vaccination plans.21 Across South America, the FMD situation varies. Several countries have achieved FMD-free status by employing either vaccination programs or strict biosecurity measures. However, Venezuela's official FMD status remains undefined.14 Bordering Venezuela, a country with ongoing FMD outbreaks, puts Brazil and Colombia's FMD-free status at risk. This concern materialized in 2017-2018, with FMD outbreaks occurring near their shared borders. Furthermore, the previous study identified a new serotype A lineage in Egypt that is closely related to Venezuelan strains and the A24 Cruzeiro reference isolate. Notably, this strain was detected in only a single Egyptian farm, and subsequent surveillance and analysis revealed no further infections with this specific virus. Phylogenetic analysis confirmed the close relationship of the Egyptian isolates to the Venezuelan and Colombian strains, while structural protein analysis categorized them as the A24 Cruzeiro prototype and the EURO-SA topotype. This highlights the potential for viral transmission across trade routes and emphasizes the need for ongoing surveillance and rapid identification of FMD strains.8) Successful management of FMDV relies heavily on the availability of effective vaccines.20) These vaccines are chosen based on a three-pronged approach: aligning their genetic makeup with circulating viruses through genome sequencing, assessing their cross-reactivity with bovine postvaccinal serum, and ensuring sufficient quantity and high quality of vaccine.22) Faced with multiple FMD serotypes offering limited cross-protection, the need for polyvalent vaccines with broader coverage is critical.23) This study was aimed to identify the cross-protection of the current vaccines against recent circulating field isolate FMDV-A-Egy-AHRI-RL385-Ven-2022.
Calves belonging to groups II and III received subcutaneous inoculations with FMDV vaccines. For calves vaccinated with the local commercial vaccine (batch 1), the humoral immune response against FMDV strains FMDV-A-Egy-AHRI-RL385-Ven-2022 and A/EGY/1/2012 (A Iran-05 lineage) was evaluated using VNT and titers of 0.36 and 2.04 log10, respectively, were obtained. In contrast, the humoral immune response induced by the imported vaccine (batch2) against the FMDV strains FMDV-A-Egy-AHRI-RL385-Ven-2022 and A/EGY/1/2012, as assessed through VNT, demonstrated titers of 0.42 and 2.4 log10, respectively, meeting the minimum protective virus-neutralizing antibody titer of ≥1.65 log10.14,20
The r1-value of the recently isolated FMDV-A-Egy-AHRI-RL385-Ven-2022 strain was examined and calculated. The obtained r1-values were 0.176 and 0.175 for vaccine batches 1 and 2, respectively. According to OIE guidelines,14 r1-values exceeding 0.3 indicate a close antigenic correspondence between the vaccine strain and the field isolate, suggesting the potential for the vaccine strain to offer cross-protection against the field strain. Conversely, r1-values less than 0.3 indicate a lack of cross-protection. These findings align with the antigenic composition, indicating that the nucleotide composition of the Egyptian isolates was 24.7% (A), 18% (T), 30.30% (C), and 27% (G). When comparing the nucleotide sequences of recent Egyptian isolates from 2022 to the prototype strain of EURO-SA, the transitional substitution rate was 66.14%, and the trans versional substitution rate was 33.86%.8
The protection level findings of the local commercial inactivated FMDV vaccine (batch 1) indicated 100% against the homologous A/EGY/1/2012 strain, however, it displayed no detectable protection 0% against FMDV-A-Egy-AHRI-RL385-Ven-2022 strain. Furthermore, with the imported inactivated FMDV vaccine (batch 2) indicated 100% protection against A/EGY/1/2012, but exhibited only limited protection 20% against FMDV-A-Egy-AHRI-RL385-Ven-2022. Per OIE recommendations,14) a suggested threshold for vaccine potency acceptance in regular vaccination regimens is 75%, corresponding to 3 PD50. Interestingly, another study reported that in comparison to two different vaccine batches (FMDV O PanAsia 2), they were assessed through a challenge test using the FMDV O-EA3 strain, revealing protective levels of 100% and 80%, respectively.24 The periodic emergence of new variant FMD viruses renders the current vaccine inefficient, therefore, the regular selection of vaccine strains, either through in vivo or in vitro methods, becomes an essential requirement to ensure the use of appropriate and effective vaccines.25
Conclusion
The currently available local and imported commercial inactivated FMDV vaccine batches (A Iran-05 lineage) have been determined to be impotent and ineffective against the present circulating FMDV-A-Egy-AHRI-RL385-Ven-2022 (EURO-SA lineage). It is strongly recommended to enhance the existing vaccines by including the isolated variant alongside the current formulation.