INTRODUCTION
X-ray Diffraction (XRD) is a technique used in qualitative and quantitative analysis of mineral phases in polycrystalline samples. The Rietveld method, used for structure refinement, can also be employed for quantitative phase analysis by using a simple relationship between the refined scale factor of each phase and its weight fraction in a multi-phase mixture. This method is currently known as quantitative analysis by the Rietveld method and has been a significant advancement in the field of quantitative phase analysis (Cullity & Weymouth, 1957; Hill & Howard, 1987; Bish & Howard, 1988; Ramírez, 2016).
The Rietveld quantitative analysis method is an efficient analytical procedure that uses all the information contained in the diffractogram without the need for standards or calibration curves. The only requirement is a reliable structural model of each phase to be quantified. If used on high-quality diffraction data, it can quantify crystalline phases with a weight fraction below 1,0 % and detect amorphous material in a multi-phase mixture by adding a known internal standard (Gualtieri, 2000; Ramírez, 2016).
Quantitative analysis of samples with multiple phases becomes more complex as the number of phases and the nature of the sample increases. Clays are an example of a sample that presents difficulties for quantification due to their composition, degree of variation in structural order, and tendency to preferentially orient. These challenges can also be caused by the scarcity of the content to be quantified in relation to the total sample, which is masked by the presence of other phases (Moore & Reynolds, 1997; Brindley, 2015; Merendón-Barcina, 2016; Ramírez, 2016).
In Cuba, the X-ray Diffraction technique has been used for the quantification of mineral phases, but the description of identification and quantification protocols has been poor in published literature. Often, subprogram routines are used for phase identification, and XRD operators do not always have knowledge of the crystalline matter they are working with. Additionally, most published studies focus on the qualitative analysis of samples rather than their proper quantification. In particular, there is a scarcity of studies in the field of clays (Labrador et al., 2006; Chang-Rodríguez et al., 2016; Merendón-Barcina, 2016; Ramírez, 2016).
The aim of this research is to characterize the influence of geological genesis and structural degree on the quantification of clay phases using the Rietveld method and an internal standard. Two clay deposits with different geological genesis, Cayo Guam (CG) and Dumañuecos (DM), were selected in order to observe the mineralogical characteristics and variations in structural disorder according to their geological origin. Cayo Guam is characterized as a weathering crust with a high degree of structural disorder due to weathering, while Dumañuecos is of hydrothermal origin, with higher purity and crystallinity.
MATERIALS AND METHODS
Geological environment
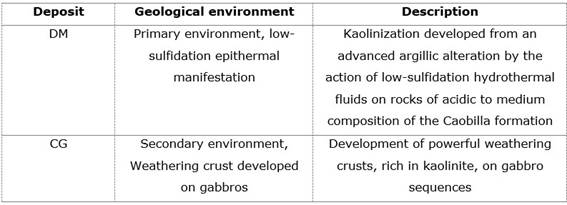
The selected deposits were divided into two groups according to the kaolinization environment. Primary environment referring to those formed by hydrothermal processes and secondary environments formed by weathering (Velde, 1995; Brigatti et al., 2006; Dill, 2016). The fundamental aspects of the selected mineral manifestations are related in Table 1.
Selection of the internal standard
In this study, an internal standard of alumina (Al2O3) was selected for the quantification of clay phases using the Rietveld method. The internal standard was subjected to X-ray diffraction to determine its purity and it was observed to have a simple structure with well-defined Bragg peaks, which validated its use as an internal standard. The minimum amorphous content of the initial material was eliminated by calcination at 1000 ºC for one hour before its use as an internal standard in the quantitative analysis of clay phases.
Sample preparation for X-ray diffraction and addition of the internal standard
The samples were wet ground manually using an agate mortar, using approximately 4 g of material in all cases, previously homogenized and quartered. The grinding was carried out for 15 minutes, in various directions, to avoid preferential orientation of the crystals. 5 ml of alcohol were added every 5 minutes until a paste was obtained, which was then dried in an oven at 80-90 °C for 24 hours. A 90 µm sieve was used for particle size control, obtaining a 5 % retained. Subsequently, 20 % by weight of each sample was replaced with the internal standard of alumina (Al2O3) and the material was homogenized in the agate mortar, adding alcohol to form a paste, which was then dried in an oven at 80-90 °C for 24 hours.
X-ray diffraction analysis
X-ray diffraction analysis was performed at UPL CEDINIQ, where a total of 10 samples for each deposit were delivered, a unitary composite sample was prepared to represent to Cayo Guam and Dumañuecos. Measurements were performed using a Panalytical X'Pert³ Powder Diffractometer using CuKα radiation and a divergence slit of 0,5°. The samples were analyzed between 5 and 80° (2θ), with an angular step of 0,008°, a time per step of 30 seconds, and without a nickel Ni filter. The diffraction patterns were processed with X Pert HighScore Plus software (2004).
X-ray fluorescence analysis
The samples for X-ray Fluorescence (XRF) were prepared in the physical laboratory of the Karlsruhe Institute of Technology. They were dried at 40 °C for 24 hours, then pulverized in a ring mill to obtain a particle size smaller than 5 microns. 2 g of previously homogenized and quartered sample were taken and calcined for 2 hours at a temperature of 950 °C. The resulting calcined material was placed to cool in a glass desiccator. The fused bead method was used for analysis. Loss on ignition was calculated at the same temperature range. It should be noted that in each of the preparations, adequate homogenization and quartering of the samples were ensured to ensure the representativeness of the chemical and mineralogical analyses.
For the quantification of the amorphous phases, an Al2O3 internal standard, supplied by the laboratory, was used and 20 % by weight of each sample analyzed was replaced.
Sample preparation for Simultaneous Thermal Analysis
The previously crushed and dried samples at 105 °C for 24 hours were analyzed in a NETZ 5CH unit for Simultaneous Thermal Analysis, model STA 409. The temperature was measured from room temperature to 1 000°C at a rate of 10 °C/min. A synthetic oxygen atmosphere was used.
RESULTS AND DISCUSSION
Results of the chemical and mineralogical analysis
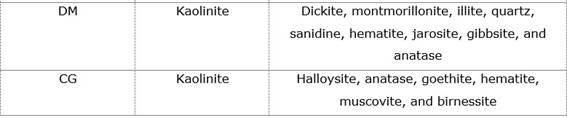
Table 2 shows the results of the X-ray diffraction analysis for each deposit, where the main clay phases and accompanying minerals identified are established.
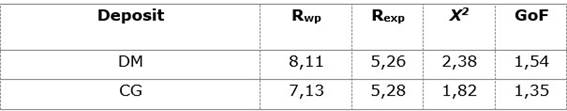
In the diffraction pattern of the Dumañuecos deposit, narrow peaks with high intensity, good resolution, and no background noise can be observed, being a direct criterion of a good crystalline structure. In the case of Cayo Guam broad, poorly defined peaks with considerable background noise are present, indicating a clear structural disorder of the sample (Figure 1). Based on this, a kaolinite phase with turbostratic disorder was added, allowing for a better fit of the model according to (Ufer et al., 2004). The Rietveld fitting parameters are shown in Table 3, with good fitting values for the accepted paragenesis observed in all cases.
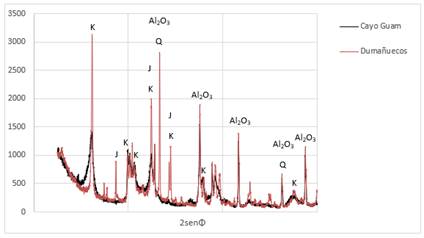
Figure 1. Overlayof the diffraction patterns of the samples, main peaks: K-kaolinite, J-jarosite, Q-quartz, Al2O3-internal standard.
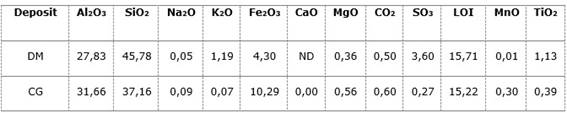
Table 4 shows the chemical results, congruent with a weathering crust like Cayo Guam, where there is an enrichment of phases rich in iron oxides and hydroxides, mainly caused by the breakdown and oxidation of pyroxenes in the gabbro. The high contents of Al2O3 are related to the decomposition of plagioclases.
On the other hand, Dumañuecos has more discrete iron contents, while having significant values of alumina and silica. Potassium is consistent with the existence of sanidine-type phases, related to the hydrothermal process.
Table 4 Values of the oxide contents of the chemical elements of the samples from the selected deposits (wt %)
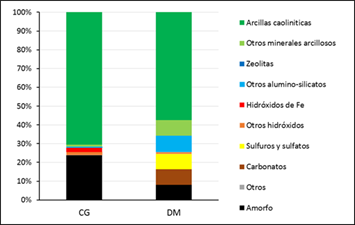
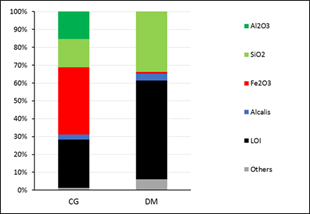
In order to achieve a more objective analysis in complex systems such as clay deposits, from a mineralogical point of view, it is proposed to group the phases into mineralogical classes based on the Strunz classification of 1938, with modifications aimed at grouping into mineralogical classes. Additionally, this classification has been widely used in the scientific community and is a useful tool for standardizing the nomenclature of minerals and their classification (Figure 2).
This type of analysis allows to determine that the Cayo Guam deposit is dominated by the presence of kaolinitic clays, constituting around 70 % of the sample. The weathering process has been so strong that all the primary material has been kaolinitized or transformed into oxides. At the same time, there is at least 5 % of clay minerals that do not belong to the kaolinite group, such as micas and iron hydroxides, mainly goethite. The amorphous phases correspond to 25 % by weight of the sample.
In Dumañuecos, clays occupy more than 50 % of the deposit, with a development of other phases, mainly sulfide phases representing 10 %, a distinctive factor in hydrothermal clay deposits where there is the presence of APS minerals (Aluminum Sulfates and Phosphates). The amorphous phase corresponds to 8 % by weight of the sample. Once again, the difference in amorphous contents between both deposits is due to the effects of weathering, with a close interrelation with rainfall, vegetation, and climate parameters in general.
Characterization of the clay fraction
Special attention was paid to the mineralogical composition of the clay phases, for which preparations with preferred crystal orientation were made (Figure 3), with heating in a glycol atmosphere and calcination at 550 °C, the latter to collapse the peaks of the minerals in the kaolinite group. The combination of these preparations allows for precision in the paragenesis of the clay phase.
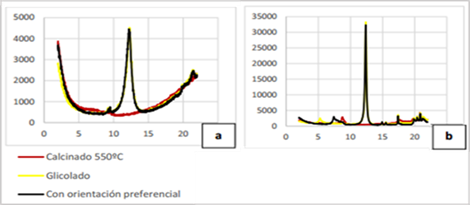
Figure 3. X-ray diffraction patterns with preferred crystal orientation of: a) Cayo Guam, b) Dumañuecos (yellow-glycolated, red-burned 550 ºC, black- with preferred orientation).
In the case of Cayo Guam (Figure 3a), a simple clay paragenesis is observed, composed of phases in the kaolinite group, which do not swell in a glycol atmosphere and collapse at 550 °C.
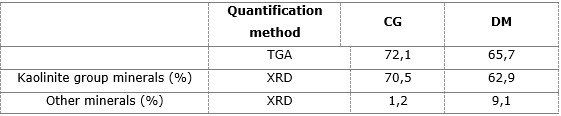
The Dumañuecos deposit contains almost 10 % of clay minerals, such as illite and montmorillonite. It should also be noted a slight trend towards over-quantification of clay minerals in the kaolinite group as determined by TGA compared to the (potentially more accurate) results obtained from Rietveld analysis, as shown in Table 5, which shows the quantification results by both methods and establishes the values obtained in each case.
Dumañuecos (Figure 3b) shows a similar trend to Cayo Guam in terms of the pronounced collapse of the peaks related to the presence of minerals in the kaolin group. Montmorillonite exhibits swelling in a glycol atmosphere up to 16 Å with collapse at 10 Å in the calcined sample. Apparently, this behavior is related to interstratifications with illites.
Quantification and characterization of amorphous
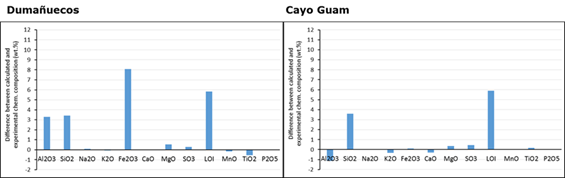
To determine the content and composition of the amorphous phase, the experimentally calculated chemical composition and the composition calculated from the obtained paragenesis were used. A discrepancy was observed between the values of the chemical elements obtained and the calculated values, suggesting the presence of an amorphous phase. Further analysis was carried out to determine the composition and amount of the amorphous phase present in the sample (Figure 4).
In the Cayo Guam deposit, the amorphous phase is complex, consisting mostly of alumina and iron oxide, which suggests that the weathering process has caused some of the iron oxide to end up in the amorphous phase. There is also a small portion composed of alkali earth metals and another portion of non-structural water.
In Dumañuecos, the amorphous phase represents 8 % of the total sample and is mainly composed of alumina (30 %). There is a small proportion of the amorphous phase that consists of alkali earth metals. Ignition losses are considerable and are mainly due to the presence of non-structural water and the loss of volatiles related to sulfides, sulfates, and zeolitized phases present in the sample (Figure 5).
CONCLUSIONS
It was determined that the most common elements in each cases are Al and Si, and that in the case of Fe, its content is associated with weathering in secondary environment deposits. The clay phase established in Dumañuecos are dominated by minerals of the kaolinite group, accompanied by clays phases 2:1, APS type minerals and a low content of amorphous phases. In the case of Cayo Guam deposit is dominated by minerals from de kaolinite group, accompanied by iron oxides and hydroxides and a high content of amorphous phases.
Correlations were established between Rietveld refinement, geological context, and mathematical model fitting, using the geological information and determined and estimated chemical compositions.
Structural and particle size differences were established that affect proper quantification and are related to the degree of weathering in each deposit.
It was determined that Cayo Guam has a lower degree of crystallinity and greater structural disorder compared to Dumañuecos, as evidenced by the peaks obtained in the diffraction analyses and determined by the formation processes of each deposit, with the level of weathering being the main cause of these results.