Introductions
Life and career
Very little seems to be known about the life and career of Marie-Auguste-Antoine Commaille (Frison, 1876; Barroux, 1961). He was born on March 9, 1826 in Saulieu, a commune in the Côte-d'Or department (France) and died on May 2, 1876, in Marseille. He served seven years in the French Army while studying pharmacy. After receiving his degree of bachelier ès-Lettres he became pharmacien militaire (military pharmacist) in 1856 and pharmacist the following year (Commaille, 1857b). Within the frame of his service he worked at several military hospitals, e.g. Val-de-Grâce, Invalides, French military hospital in Rome, Marseille, etc. in different positions, e.g. pharmacien-aide major de 1er classe, pharmacien-major de 1er classe, head pharmacist at Marseille, etc. He also served in several academic positions, among them, professor of chemistry at the École de Pharmacien Militaire and substitute professor of pharmacy at the École de Médicine in Algiers. In 1866 the Faculty of Sciences of Paris awarded him his diploma of docteur ès-Science, after successfully defending a thesis about albuminoidal substances (Commaille, 1866a). He spent the last part of his life in Marseille (1868-1876). He was appointed chevalier of the Légion d'Honneur and was awarded a gold medal by the Académie de Médicine for his work on caffeine (Frison, 1876; Barroux, 1961).
Scientific work
Commaille wrote about 60 papers in the areas of analytical, inorganic, and organic chemistry, physiology, toxicology, and plant principles. In addition to the subjects discussed below, Commaille studied the preparation of potassium nitrate from the stems of Algerian tobacco (Commaille, 1856), the preparation and analysis of the tincture of iodine (Commaille, 1857a, 1859); the dyeing material present in the tubercles of branched asphodel (Asphodelus ramosus) (Commaille, 1858); and the possible presence of copper and aluminum in vegetables (Commaille, 1863a b); he analyzed the milk of cat (Commaille, 1866b); determined the amount of soluble carbohydrates present in the juice of melon and watermelon (Commaille, 1869c); the action of ammonia on phosphorus (Commaille, 1869d, 1871b); studied the diffusion of albuminoidal liquids in contact with distilled water (Commaille, 1871a), developed a process for separating cholesterol from fatty matter (Commaille, 1875b), analyzed a suppurating jaundiced pancreas (Commaille, 1876a), studied in detail the viscous (mucous) fermentation (Commaille, 1876b), etc. With Eugène Millon (1812-1867) he studied the chemistry, purification, dosage, and the value of the equivalent of copper (Millon & Commaille, 1863b c d, 1864a), etc.
In what follows care has to be taken to consider that Commaille used the old values of atomic masses (C = 6, O = 8, hydrogen monatomic and water = HO).
Atractylis gummifera, L.
Atractylis gummifera L. (Asteraceae) is a thistle distributed worldwide but especially abundant in the Mediterranean regions. It grows commonly in dry areas; the juice of the rhizome is poisonous but both therapeutic and toxic properties of this plant have been reported. In popular medicine, Atractylis gummifera has been used to treat several conditions including intestinal parasites ulcers, snakebite poisoning, drowsiness, antipyretic, diuretic, purgative, and emetic properties. The active ingredients the plant is known to cause severe hepatitis followed usually by fatal liver failure.
In 1854 Commaille sent a note to the Académie of Sciences reporting several cases of children that had died as consequence of eating the roots of Atractylis gummifera (Commaille, 1854). The note included a medical description of the physiological damage observed in the victims before their death. The results of the autopsy indicated poisoning by a toxic substance acting as irritant and stunning of the ganglion respiratory system (lung paralysis) and the ganglion ophthalmic system. These results induced Commaille to search if the plant contained a substance toxic to living animals. After several exploratory experiments he found that maceration of the roots of Atractylis gummifera with pure water produced an extract that rapidly killed cats and that the autopsy of their bodies showed disorders very similar to those presented by the children, particularly, dilation of the pupils. The extract was found to contain a particular acid that Commaille named atractylic acid, which formed alkaline, alkaline-terreous, and metallic salts (Commaille, 1854).
Several years later Edmond Lefranc carried an extensive research on the plant, reporting its botanical characteristics and its chemical and toxicological properties (Lefranc, 1866, 1868a b, 1869). Leblanc wrote that the scientific literature mentioned that the roots of the plant contained a principle that was toxic and mortal to humans but the other parts of the plant provided a safe food matter. Chemical analysis of the roots indicated that they contained inulin and several sugars, coloring matter, a kind of caoutchouc, a toxic narcotic principle, asparagine, albuminous matter, inorganic salts, water, etc. The toxic matter dissolved easily in milk; an infusion prepared from the decoction of 100 to 150 g of fresh roots, dissolved in 500 g of milk, resulted in the prompt death of a dog. Medical information on human victims suggested that a dose of about 100 g was mortal. The roots lost their toxic power after exposure to the atmosphere for several days or long cooking at about 100 oC (Lefranc, 1866). Lefranc added that the roots contained an immediate product constituted by a potassium salt that strongly reddened litmus paper and contained the elements of sulfuric acid attached to a saccharide group belonging to the levulosides (Lefranc, 1868a b). This natural potassium atractylate appeared as prismatic needles, levorotatory, colorless and odorless, with a bitter taste, and soluble in water and diluted alcohol. This compound could be heated to 120 oC, without decomposition. At 160 oC it swelled, blackened, and released vapors of valeric acid accompanied by traces of a rose colored liquid. The potassium atractylate seemed to be composed of the elements of sulfuric acid, of a ternary molecule of formula C60H52O20 originating from valeric acid, a fermentable sugar, and a resin, suggesting that the compound belonged to the type vinic acids. This assumption was supported by the fact that treated with barium chloride it behaved as a sulfovinate (derivative of ethyl sulfate) instead of a sulfate. The acid could be separated by treating lead basic atractylate with a stream of hydrogen sulfide, followed by precipitation of the excess of hydrogen sulfide with lead sub-acetate. The acid was very soluble in water; the solution was colorless and odorless, with a sweet and bitter taste. It had three acid groups that yielded the three possible salts, the natural atractylate belonging to the series (2KO + H).(atractylate), and hence of general formula S4O12,C60H52O20.2KO+H (Lefranc, 1868a b, 1869).
These publications lead to a strong polemic between Lefranc and Commaille, the latter claiming that he had been the first to identify the presence of an acid in Atractylis gummifera, and given it the name atractylic acid (Commaille, 1869a). Lefranc answered that he believed that the acid that Commaille had separated was not atractylic acid because it would have been destroyed under the conditions that Commaille had conducted his experiments. Commaille answered that he had conducted his experiments below the boiling point of water, a temperature below the decomposition temperature reported by Lefranc. In addition, Lefranc had reported the crystalline acid could be heated to 120 oC, without decomposition (Lefranc, 1869). Commaille also criticized Lefranc's contention that atractylic acid belonged to the gender vinic acid and to the group glucosides or saccharides. Sulfovinic acid was not a glucoside and none of the acid glucosides belonged to the group vinic acids. Atractylic acid was certainly a glucoside. According to Commaille, the natural potassium atractylate was exactly equal to potassium myronate (present in mustard seeds). Both contained sulfur and potassium, the acid was non-crystallizable; their potassium salts were colorless and odorless and had a bitter taste. They were very soluble in water and alcohol and insoluble in ether. The acids decomposed by distillation; and all the myronates and corresponding atractylates were soluble in water and crystallizable (Commaille, 1869b).
Today we know that the toxicity of the roots of Atractylis gummifera is due to the presence of atractyloside and carboxyatractyloside, two diterpenoids that inhibit mitochondrial oxidative phosphorylation (Daniele et al., 2005).
Coffee
Commaille published two papers on the subject, one on the solubility of caffeine and its melting point (Commaille, 1875a), and another on the composition of coffee (Commaille, 1876c). Several scientists had studied coffee, among them, Antoine-Alexander Cadet de Vaux (1743-1828), Charles-Louis Cadet de Gassicourt (1743-1828), Richard Chenevix (1774-1830), and Anselme Payen (1795-1871) (Commaille, 1876c). Cadet de Vaux and Cadet de Gassicourt had only reported some general characteristics of the grains and a very qualitative description of its possible composition, without identifying the active components. Chenevix had separated a new vegetable principle, different from tannin or any other known vegetable principle. The only common property it had with tannin was its affinity for tin oxide (Chenevix, 1802). Payen had gone further and determined the general composition of coffee (Payen, 1849). His results indicated that normal coffee contained, approximately, by weight, 34% cellulose, 12% hygroscopic water, 10 to 13% fatty matter, 15.5% glucose, dextrin and an undetermined vegetable acid, 10% legumin and casein, 3.5 to 5% of potassium chloroginate and caffeine, 3% of a nitrogenous substance, 0.8% free caffeine, 0.001% solid essential oil, 0.002 aromatic essence, and 0.697% of mineral salts. Payen also isolated caffeine and wrote that it appeared as prismatic crystals, white and brilliant, fusible and volatile without leaving a residue. Elemental analysis indicated that it contained, by weight, 50.855% carbon, 5.085% hydrogen, 30.000% nitrogen, and 14.060% oxygen, corresponding to the formula C16H10N4O3. Payen mentioned that the coffee extract had a rich green coloration caused by an acid that he named chlorogenic acid (Payen, 1849). This acid appeared as a double salt of potassium and calcium (today we know that chlorogenic acid is present as an ester of caffeic and quinic acids, and that it is the major polyphenolic compound in coffee).
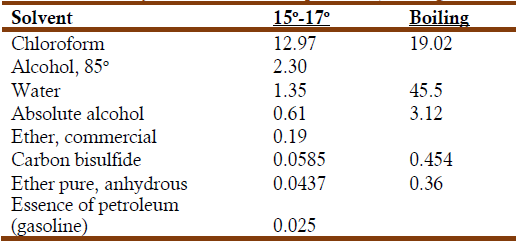
According to Commaille, the determination of caffeine was a difficult task; all the available procedures took very long (Commaille, 1875a). Commaille tested all of them, particularly the one suggested by Gerardus Johannes Mulder (1802-1880) (Mulder, 1838) and modified by Rudolph Weyrich (Weyrich, 1874). This procedure worked well until the stage where it was necessary to filtrate the hot green precipitate formed by the coffee decoction. This step began to slow down until it became practically inapplicable. After much testing, Commaille modified it as follows: He took 5 g of powdered coffee, passed through a sieve of silk, No. 60, mixed it with 1 g of calcined magnesia, and left the resulting paste to rest for twenty-four hours in contact with air. During this time the paste turned yellow and then green. When spread upon a saucer placed upon boiling water it turned into a green solid mass, which was triturated and sifted. The green powder was treated several times with anhydrous chloroform, as suggested by E. Lieventhal (Lievental, 1872). The almost colorless residue was found to consist of fatty and waxy matters, and of caffeine. It was then mixed and heated with water and 10 g of powdered glass. This separated the caffeine from the fatty material that floated over the water phase. Evaporation of the water solution left a white crystalline residue of caffeine, which was then dried. Commaille determined the solubility of anhydrous caffeine in different solvents and reported his results in the following table (Commaille, 1875a):
No one had studied coffee as such after Payen. For this reason, Commaille decided to study this commodity through its many aspects: commercial sources, toasting, chemical composition, etc. (Commaille, 1876c) He thought he was in a particular advantageous situation because Marseilles had the largest coffee market in France, and also of the world largest, and thus he had access to many coffee specialists. His general purpose was to find if by means of one or more of the components of coffee (caffeine, tannin, cinders, albumen, etc.) it was possible to explain the notable differences that the gourmets found in the drink, that is, to use analytical means to classify the diverse varieties of coffee. Commaille believed that the results published by Payen did not seem reliable particularly since he had not given any details regarding the analytical procedures he had used to determine the amounts of glucose, dextrin, albumen etc. For this reason, he decided to repeat the quantification of several of the main components of coffee, being aware that the numerical results would vary with the nature of the grain. Commaille used Mysore coffee (originating from India) that had been ground in a porcelain mortar and sieved. It contained 13% weight water and weighed 700 g per liter (Commaille, 1876c).
In the first set of experiments Commaille determined the amount of water extracted by cold and boiling water (Commaille, 1876c). The resulting shady greenish liquid from the cold extraction was filtered, evaporated, and dried at 110o-120 oC. The resulting residue constituted 24.97% of the original material and was very bitter and hygroscopic. Calcination reduced it weight to 13.20% of the original one. The cinder was white, hard to burn, and contained a large amount of phosphates. The final count indicated that 100 g of cold water dissolved a weight of 3.296 g of mineral matter. The experiment with boiling water left an insoluble residue amounting to 37.2% of the original weight and containing 1.23% of cinders, and very little phosphates. This was an important the result: boiling water dissolved most of the phosphates (Commaille, 1876c).
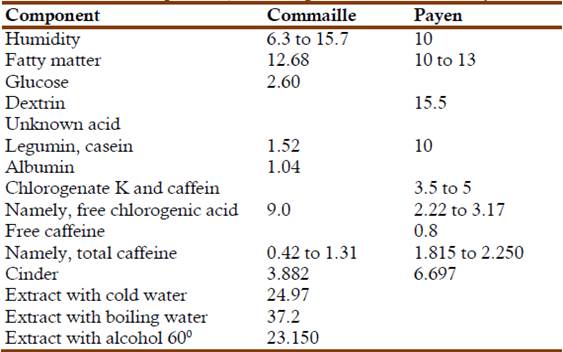
The next step was determination of the amount of albuminous matter by means of five different experiments, four with cold water and two with warm water. In the first experiment 100 g of Mysore coffee were extracted, successively, by ether, alcohol of 60o, alcohol of 90o, and then by cold water. The resulting water phase was loaded with nitrogenous matter that alcohol precipitated as cheesy flakes. The next experiments involved maceration with different amounts of cold water, with alcohol, warm water, boiling water and precipitation with acetic acid, etc. The final results indicated that the sample contained 1.040% legumin and 1.520% albumin, for a total of 2.560%. In following experiments 100 g of coffee were extracted with ether; evaporation of the ethereal extract left a residue of 13.112 g. The residue was then extracted with dry ether, exempt of alcohol. The next extract contained only fatty oil. Evaporation to dryness left a final residue of 12.681 g. Commaille's paper described in detail the separation and analytical process for determining the mayor components of coffee. He ended this paper with the following table comparing his results with those Payen (Commaille, 1876c):
Table 2: Coffee composition, according to Commaille and Payen
Commaille tried unsuccessfully to determine the melting point of caffeine, but failed due to the uncertainty of his thermometers at high temperatures (Commaille, 1876c).
Milk
In 1864 Eugène Millon (1812-1867) and Commaille reported the discovery of a new albuminoidal substance present in the milk of cow (Millon & Commaille, 1864c). They mixed at 10 oC a small amount of acetic acid with cow milk diluted in four times its volume with water, separated the coagulum of casein, heated the filtrate to boiling and noted the precipitation of a new coagulum having the external appearance of albumen and the same amount of nitrogen (15.6% weight). The new coagulum secreted a small amount of whey that was found to contain the new albuminoidal substance, which they named lactoprotein. Treatment with Millon's reagent proved it was a protein. According to Millon and Commaille lactoprotein was not coagulated by heat, nitric acid, mercuric chloride, and the combined action of heat and acetic acid. Lactoprotein was seen to form an insoluble compound with a nitric solution of mercuric oxide; this property was used to separate it from the whey. The resulting precipitate was found to be white, amorphous, and insoluble in water, alcohol, and ether, and turning yellow or red upon desiccation; it was purified by successive washings with water acidulated with nitric acid, pure water, alcohol, and ether; and then dried. Lactoprotein was also found to be present in different amounts in the milk of other animals (human milk, goat, ewe, and ass). One liter of cow milk yielded between 2.90 to 3.49 g of lactoprotein. The formula of the protein, C36H25N4O10, indicated that milk contained the product of the oxidation of the protein combined with ammonia. It reacted with mercuric oxide nitrate to form a combination of lactoprotein with mercuric oxide (Millon & Commaille, 1864c).
In the following paper, Millon and Commaille reported the full analysis of milk (Millon & Commaille, 1864d). They stressed the need to dilute the milk with four times its volume with water, previous to its analysis; otherwise the precipitation of albuminoidal substances would be very slow, of hard work, and essentially impractical. Thus, they diluted 20 cm3 of milk in 80 of water and then added 5 to 6 drops of acetic acid. As a result, the milk split into a floating coagulum and a liquid phase. The analysis of the coagulum provided the amounts present of butter and casein present, and analysis of the liquid, the amounts of albumen, lactoprotein, lactose, and salts. The coagulum was washed several times first with water, then with alcohol 40o and absolute alcohol, and finally with ether mixed with alcohol. This removed all the fatty material. The ether alcohol extract was evaporated to dryness over a water bath. The residue of butter was then weighed. The residue not dissolved by the absolute ether alcohol mixture represented the amount in casein present in the coagulum of 20 cm3 of milk. Upon drying it left a residue of white and pure casein, varying between 33.50 to 36.83 g per liter of milk. Different milk samples provided slightly different results. The whey was divided in three portions for the determination of albumen + lactoprotein, lactose, and cinders. The results indicated that 1 liter of cow milk contained 5.25 g of albumen; goat milk 6.43 g, ass milk 1.83 g, and human milk, 0.88 g. The amount of lactose, 44.64 g, was determined using Fehling's liquor and that of cinders, 7.03 g, by calcination in a platinum capsule (Millon and Commaille, 1864d).
The three following papers discussed the properties of casein (Millon & Commaille, 1865a b c). Millon and Commaille wrote that filtering milk that had been diluted with four times its volume with water, left on the filter a cream formed of diverse globules. Washing this deposit with alcohol, ether, or carbon disulfide to eliminate all the fatty material, left on the filter a white, heavy, and floury material very similar to the casein that acetic acid precipitated from milk. This simple procedure left two caseins, one insoluble and maintained in suspension, and another, soluble, and precipitated under cold by acetic, sulfuric, nitric, and oxalic acids (Millon & Commaille, 1865a). Initially Millon and Commaille did not know if these two substances were isomeric or had different compositions. Nitrogen analysis indicated that they were different, the insoluble casein contained 14.7% weight nitrogen, and the soluble one 17.18% weight. The similar aspects of both caseins suggested Millon and Commaille that they were the same basic substance, each combined with a different material. The experimental evidence confirmed this assumption. Millon and Commaille found that casein combined with a large number of mineral and organic acids, among them, HCl, chloroplatinic, sulfuric, chromic, nitric, phosphoric, arsenic, and oxalic acid. These combinations were general insoluble and typified by the formation of a characteristic coagulum. Acids, particularly tartaric and citric, redissolved this coagulum. Free acids, employed in excess, displaced the combined one. Thus, for example, addition of nitric acid to a solution of sulfuric (oxalic, phosphoric, etc.) casein in NaOH precipitated the nitric casein. This is the property that Millon and Commaille used to prepare the 9 different acid combinations of casein, listed in their paper (Millon & Commaille, 1865a).
The following paper described the preparation of the additional acid caseins with HI, acetic perchloric, and thiocyanhydric acids. All of them were decomposed by water (Millon & Commaille, 1865b). Millon and Commaille wrote that casein was more soluble in basic water (NaOH, KOH, ammonia) than that of egg or milk albumen (Millon & Commaille, 1865c). This led them to study the affinity of casein for the bases. They illustrated their results by grinding casein with magnesia, followed by dilution in water and separation of the liquid. Addition of the liquid to concentrated alcohol precipitated a white precipitate. Analysis of the same showed that it was composed of 1 equivalent of casein, 2 of magnesia, and 4 of water. A similar result was obtained by repeating the procedure using a mixture of 2 oxides, for example cupric oxide and magnesia. The resulting compound contained 1 equivalent of casein, 1 of cupric oxide, and 2 of magnesia. Using other oxides, for example, calcium oxide, potassium oxide, barium oxide, sodium oxide, etc. produces similar compounds, although in different molecular ratios (Millon & Commaille, 1865c).
Commaille also studied the presence of creatinine in putrefied whey (Commaille, 1868). For this purpose, he introduced filtered whey in a flask covered only by a sheet of paper. After some time, the whey began to ferment and eventually to putrefy, showing the presence of a large number of microorganisms in all the liquid. Finally, the liquid became dark brown, all the microorganisms were dead, a heavy layer of spores was present at the bottom of the flask, and an infection odor replaced the mildew one. Commaille evaporated the liquid and extracted the residue successively with alcohol of 85o, 90o, and 950, and evaporated the extract to dryness. The solid residue showed the presence of abundant mineral salts. Calcination left a white salty residue that was dissolved in water and treated with silver nitrate. The resulting white precipitate was extracted with boiling water and yielded white needles that Commaille assumed were creatinine nitrate. The alcoholic extract was evaporated and left a residue of rectangular prismatic crystals. These crystals were soluble in water and alcohol, and insoluble in ether. Treated with silver nitrate they yielded the double nitrate of silver and creatinine and treated with zinc chloride they precipitated small needles, composed of the double chloride of zinc and creatinine. Commaille believed that the creatinine, C8H7N3O2, present in the whey originated, without doubt, from the creatine, C8H9N3O4.2HO, that existed in milk. Hence, it behaved like urine, which abandoned in the air, contained, at the end, creatinine instead of creatine (Commaille, 1868).
Chemical composition of albuminoidal substances
As mentioned above, Commaille doctoral the thesis was devoted to the determination of the chemical constitution of albuminoidal substances (as known and named at his time) (Commaille, 1866a). After some general considerations about these materials he proceeded to describe the properties of the following ones, present in: (1) flour (gluten fibrin, gluten casein, glutin, mucine, and flour albumen); (2) chicken eggs [albumin, vitelline, and pexine (the albumin coagulated by heat)]; (3) bitter almonds (one coagulable by cold and another by heat); (4) leguminous seeds; (5) milk (casein, milk albumin, and lactoprotein); (6) blood (fibrin, globulin, and serum albumin); (7) muscle flesh (musculin and oposin); and (8) urine. For example, Commaille wrote that an aqueous solution of flour albumen was coagulated by heat, precipitated by an excess of HCl, and by mercuric chloride, and was soluble in alkaline water. Gluten casein was soluble in a very dilute solution of HCl and was not precipitated by mercuric chloride. Commaille summarized all his findings in a table reporting for each material its solubility in water, alcohol, dilute HCl, solutions of potassium nitrate, magnesium sulfate, and acid solution of mercuric chloride, and acetic acid. The table contained also the effect of heat and the rotatory power (Commaille, 1866a).
Mustard
In 1870 Commaille published a paper about white and black mustard, giving a short summary of what was known about these two products (Commaille, 1870). White mustard, Sinapis alba, had medicinal properties that were difficult to explain, and the information about its chemical composition left much to be desired. It was known to contain sulfur in a form that released readily thiocyanic acid [discovered by Rinck (Rinck, 1806)] containing 54% of its weight in sulfur and very poisonous in its free state. This acid was combined with the base sinapine, C32H24NO10, not containing sulfur, and from which it split easily into sinapic acid, C22H12O10, and a new base, sincaline (choline), C10H14NO2. Black mustard, Sinapis nigra (Brassica negris) differed from white mustard by the absence of sinapine, by its thiocyanic acid being combined with the alcoholic radical allyl, and containing much more sulfur than sinapine thiocyanate. This difference explained why the odor released by putrefying black mustard was much stronger than that from white mustard. Commaille added that these two thiocyanates were not present as such in their respective seeds; they originated when the natural principles present in the seed began to ferment in the present of water. The same phenomenon took place when the substance myrosin, present in the seed of black mustard, acted on its potassium myronate. This reaction generated the odoriferous and sapid volatile oil characteristic of black mustard (Commaille, 1870).
This information strengthened the need for further research because it was now accepted that fermentation occurred only by the action organized living bodies (microorganisms). Myrosin, the presumed ferment of mustard, did not satisfy this condition because white mustard did not contain myronic acid, explaining why it could not provide essence of mustard. Myrosin in the presence of water and sinapisine yielded sinapine thiocyanate, a principle very different from the essence of black mustard. Sinapisine, discovered in 1831 by Ossian Henry (1798-1873) and Garot (Henry & Garot, 1831), was a sulfurized substance, crystallizable and soluble in alcohol. It was a crystalloid, like myronic acid; while myrosin, which did not crystallize, was a colloid (Commaille, 1870).
Commaille added that the "purgative properties of white mustard had been explained as a mechanical action of carrying through and expulsion, a sweeping of the intestine, attributed to the presence of sulfur and to a specific action, which, considering the integrity of the mustard in the fecal matter was not very clear" (Commaille, 1870). A microscopic examination of the seeds allowed him to give a much simpler mechanical explanation: the seeds were not particularly rough; left in cold water they turned very mucilaginous. In warm water (body temperature) this process was much faster and allowed the seeds to roll one upon another very easily and induce bowel movement. Commaille added that the depurative properties of the seed were not so easy to explain because the seed seemed to be expelled in the excreta without having undergone any modification. Anyhow, he proposed an explanation based on the concepts developed by Thomas Graham (1805-1869) for the phenomenon of dialysis, taking into account that sinapisin was a crystalloid and myrosin a colloid. Membranes (like the thin perisperm of the seed) allowed crystalloids to exude easily while they retained colloids. Hence, sinapisine might be quickly carried off from the mustard seeds, although they remained intact, to be absorbed by the liquids of the digestive canal, and transferred by assimilation into the entire organism (Commaille, 1870b).
Astringent materials
Commaille defined astringent substances as those principles present in vegetables that are soluble in water, have a caustic but not bitter taste, yield with ferric oxide acetate an amorphous black, green, or gray precipitate, give or not give a precipitate with gelatin, and have no particular composition. Although these materials were produced in industrial quantities, science offered no practical procedure to determine their ratio in proportion to the inert amount of materials that accompanied them. The only exception seemed to be tannin matter, such as gallnut (Commaille, 1864). Commaille developed a rapid analytical method based on the principle developed by Millon stating that organic substances behaved in three different ways when heated in solution with iodic acid (Millon, 1845): Some were completely prevented of oxidation by iodic acid in the presence of small quantities of HCN (e.g. oxalic, formic, tartaric, meconic, citric, and lactic acids, starch, sugars, gums, etc.); others were attacked in the presence of hydrocyanic acid (e.g. proteins, acetone, gallic acid, tannin, creosote, and morphine); lastly, there were some that were not oxidized by iodic acid under any circumstances (e.g. acetic, butyric, and camphoric acids, urea, gelatin, fatty acids, quinine, caffeine, asparagine, etc.). Vegetable astringent matters belonged to the second group (Commaille, 1864).
Commaille's procedure was as follows: Take a given volume of astringent liquid, add a few drops of hydrogen cyanide acid and then a given volume of a standard solution of iodic acid, 0.5 g being usually sufficient. Boil the mixture for 15 about minutes where all the iodine set free will disappear. The liquid is now cooled and measured and then decolorized by shaking it with well-washed animal charcoal. The astringent matters combine with portions of the iodic acid, and then the remaining iodic acid is estimated as silver iodate. It is now enough to know how much iodic acid corresponds to a unit of tannin and gallic acid, to calculate the weight of these bodies in the substance analyzed. Commaille found that 1 gram of gallic acid destroyed 2.336 g of iodic acid, and 1 gram of tannin 2.320 g of the same compound. Commaille concluded his paper by giving a table containing the quantities of astringent matters existing in a number of natural products, among them, dry coffee, common wine, raw caoutchouc, Campeche tree, heaves of bushes, etc. (Commaille, 1864).
Coralline (aurin, rosolic acid)
Aurin is a dyeing compound forming yellowish or deep red crystals, discovered by Friedlieb Ferdinand Runge (1794-1867) by distillation of coal tar (Runge 1834). Commaille wrote there was a regrettable confusion regarding the names of the dyes obtained from phenol; the names rosolic acid, yellow coralline, and red coralline were applied to one or more substances obtained by diverse reactions (Commaille, 1872, 1873). His work on the subject referred to the product prepared using the procedure of Jules Persoz (1837-1927), based on the oxidation of phenol with sulfuric and oxalic acids:
2(C12H6O2) + 2SO3 + 2HO = C20H8O4 + C4H6S2O8
Phenol Corallin Parathionic acid
Commaille's experiments indicated that the best method was to react 300 parts of phenol with 200 of sulfuric acid, and 200 of oxalic at a temperature not exceeding 150 °C; at this temperature 100 parts of phenol yielded in six hours 26 of corallin, and of the oxalic acid employed 72% could be recovered from the cooled mother liquor (Commaille, 1872, 1873).
The mother liquor was concentrated by evaporation until they stopped yielding corallin. The filtrate was boiled and treated with an excess of litharge (PbO); upon cooling it gave substances, of which two were crystalline and well defined: lead parathionate and thioamylate. Commaille reported that purified lead parathionate appeared as rhomboidal plates of formula 2(C4H5O7S2).3PbO, which washed for a long time with cold or warm water, decomposed into a soluble salt crystallizing as elongated prisms of formula C4H5O7S2.3PbO.HO, and an insoluble sub-salt, white and pearly, containing, by weight, 5.50% sulfur and 73.38% lead, corresponding to one molecule of parathionate with four equivalents of base. The corresponding acid was syrupy and was not precipitated by lead acetate or baryta water (Commaille, 1872, 1873). Lead thioamylate crystallized as long silky needles, sweet and bitter, very soluble in water, soluble in alcohol, insoluble in ether, and of formula C10H11O7S2.PbO.HO. Commaille also reported the preparation of the thioamylates of barium, potassium, zinc and ammonia. Thioamylic acid was prepared by treating the lead salt with sulfuric acid, followed by addition of alcohol and precipitation of the acid as long deliquescent needles, soluble in water in all proportions, sparingly soluble in alcohol, and even less in ether (Commaille, 1872, 1873).
Commaille remarked that treating corallin with neutral or acid water, followed by treatment with lead oxide, did not produce a colored material. It was usually accepted that red corallin was an amide of the yellow, but Commaille did not agree with this idea. It was known that treating yellow corallin, both with ammonia and other bases, yielded red corallin without the help of heat and that the solution of yellow corallin in ammoniacal water yielded the same products of decomposition as the artificial red. Commaille, therefore, considered that yellow corallin was not an acid, as previously supposed, and that red corallin was not the amide of the yellow. The quantity of oxalic acid employed was far too large. Corallin yielded no definite metallic compounds, but merely colored lakes (Commaille, 1872, 1873).