INTRODUCTION
Peptic ulcer is the term used to refer to the group of ulcerative lesions of the upper gastrointestinal tract, either in the stomach or in the first portion of the duodenum (Ramakrishna and Salinas, 2007). Peptic ulcer is defined as the lesion that extends to the mucous layer and sometimes to the muscular layer of the stomach or duodenum, forming a cavity with acute and chronic inflammation around it, being this the main cause of gastrointestinal bleeding, one of the its main complications. Other complications of peptic ulcer are perforation, penetration and obstruction and all have a significant impact on the quality of life of those suffering from the disease (Ramakrishna and Salinas, 2007; Milosavljevic et al. 2011; Malik, 2022). Peptic ulcer has an incidence of 5 and 10 % in the world population (Zambrano and Roger, 2021; Malik, 2022), while in Cuba, according to the Health Statistical Yearbook (2021), it is among the top twenty causes of morbidity and mortality.
Peptic ulcer occurs as a consequence of an imbalance between aggressive factors (acid secretion, pepsin, helicobacter pylori, nonsteroidal anti-inflammatory drugs -NSAIDs-, ethanol) and defensive factors (mucus and bicarbonate secretion, blood flow, prostaglandins -PG-, endogenous protective agents such as epidermal growth factors) of the gastric mucosa, being Helicobacter pylori infection and the continued use of NSAIDs the main causes. Despite notable advances in its management and treatment, the mortality rate from complications of peptic ulcer has remained constant in recent years (Seo et al. 2016; Malmi et al. 2016; Malik, 2022).
Conventional pharmacotherapeutics for the treatment of gastric ulcer include acid secretion inhibitors (H2 antihistamines, H+/K+-ATPase pump inhibitors, anticholinergics and gastrin antagonists), cytoprotectors and antacids. Currently, the range of drugs for the treatment of gastric ulcers is very wide and diverse, depending on the specific causes that triggered it (Alfonso et al. 2014; Aguilera et al. 2016; Shah and Gossman, 2023; Vakil, 2023).
Therefore, the search for new safe and effective antiulcer therapeutic strategies continues to be a current problem.
The active ingredient beeswax alcohols (BWA-AI) (Apis mellifera) is a mixture of six high molecular weight primary aliphatic alcohols, whose main component is triacontanol, followed by hexacosanol, octacosanol, tetracosanol, dotriacontanol, and tetratriacontanol. (Mas, 2001). This mixture exerts antioxidant, gastroprotective and anti-inflammatory effects demonstrated in non-clinical and clinical studies, being safe and well tolerated (Carbajal et al. 1995; Carbajal et al. 1996; Carbajal et al. 1998; Carbajal et al. 2000; Molina et al. 2001; Menéndez et al. 2000; Menéndez et al. 2001a, b); Hano et al. 2001; Molina et al. 2005; Illnait et al. 2005; López et al. 2008; Fernández et al. 2008; Ravelo et al. 2010; Molina et al. 2011; Pérez et al. 2012, 2013; Oyarzábal et al. 2012; Molina et al. 2012; Valle et al. 2012; Rodríguez et al. 2012; Molina et al. 2015).
At the present time, it is registered as a nutritional supplement (Abexol®) in Cuba and as a functional food or alternative medicine in other countries in 50 mg tablet-form pharmaceutical. Its commercialization, both in Cuba and abroad, has a high demand. However, today this pharmaceutical formulation has several drawbacks that may limit its commercialization, such as: its limited bioavailability due to having fatty alcohols in its composition; the large size of the tablet, which implies high production costs and the increasing rejection in different countries of the use of the excipients croscarmellose and polyvinylpyrrolidone. In this sense, a new formulation of Abexol® was developed in the form of a suspension that may present different benefits, including increased bioavailability and immediacy of the pharmacological action and greater flexibility in dosage and patient satisfaction (Haim et al. 2012).
Taking into account this background, the aim of this work was to compare the effects of the BWA-AI and its formulations in form of BWA-tablets and BWA-suspension on ethanol- induced gastric ulcer in rats.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (250-300g of body weight) from National Center for the Production of Laboratory Animals, were used, which were adapted for seven days to the usual laboratory conditions (temperature from 20 to 25 0C, relative humidity of 60 ± 5 %, light/dark cycles of 12 hours) and with free access to water and food (standard food for rodents from National Center for the Production of Laboratory Animals).
The handling of the animals was carried out according to the norms established in the "Ethical Guide for the Handling of Laboratory Animals" (Havana, Cuba, 1992) and the ethical principles for the use of laboratory animals recommended in the international guidelines and in the Republic of Cuba. In addition, it was complied with chapter VIII of Decree-Law 31/2021 “On Animal Welfare” (Havana, Cuba, 2021), the regulations established by the Center for State Control of Medicines, Equipment and Medical Devices (CECMED) (Cuba): Regulation No.64/2012 “Guidelines for the Constitution and Operation of the Institutional Committees for the Care and Use of Laboratory Animals (CICUAL)”, Regulation No.39/04 “Principles of Good Non-Clinical Laboratory Practices for Health and Environmental Safety”; as well as the specific aspects reflected in the Quality Manual and Standard Work Procedures of the Center for Natural Products belonging to the National Center for Scientific Research (CNIC), Havana, Cuba. The study protocol was reviewed and approved by the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL) of the Center for Natural Products.
Treatments and experimental design
The substances to be evaluated were BWA-AI (CNIC Production Plant, Havana, Cuba) and Omeprazole (OMP) (BioCubaFarma, Cuba) in the form of acacia gum suspension, the BWA-tablet and the BWA-suspension (BioCubaFarma, Cuba).
Rats were randomized into nine groups (10 rats/group): a negative control treated with the vehicle and eight groups which the ulcer induced by ethanol (60 % in distilled water; Sigma-Aldrich): one positive control (treated with the vehicle), one with omeprazole-reference substance (20 mg/kg), and six treated with BWA-suspension, BWA-tablet, and BWA-AI at doses of 25 and 200 mg/kg.
BWA-AI doses were selected according to the range of preventively effective doses in this model (Carbajal et al. 1995; Molina et al. 2017). The OMP dose has also been effective in this model (Molina et al. 2017). All treatments were administered orally, as single doses by gastric gavage, one hour before the damage induction.
Gastric ulcer induction
The animals were fasted for 24 hours prior to the experiment, with free access to water. One hour after the administration of the treatments, the gastric ulcer was induced by the single oral administration of ethanol (60 %) a reason to 1 mL/200 g body weight (Al-Wajeeh et al. 2017).
Evaluation of gastric mucosal damage
Past one hour, the rats were sacrificed in a halothane atmosphere and the stomachs were removed. The gastric mucosal lesions were quantified by two independent blinded observers. Lesion score was defined as the sum total of lesion sizes in mm (Ohara et al. 1992).
Quantification of gastric mucus content
The collected gastric mucus was weighed in an analytical balance and the amount of mucus was expressed in mg.
Determination of lipid peroxidation and protein totals in gastric mucus
The lipid peroxidation was quantified by formation of substances reactive to thiobarbituric acid according to the technique described by Ohkawa et al. 1979; and was expressed as nmol of malondialdehyde (MDA)/protein mg. Total protein was measurement by the modified Lowry method (Marxwell et al. 1987).
Statistical Analyses
Statistical analyses were performed using Statistical software for Windows (Release 4.2, StatSoft, Inc USA). Non-parametric Mann-Whitney U test were used, establishing a priori a significance level of α = 0.05. Data are expressed as mean ± standard error.
RESULTS
Figure 1 shows representative images of the gastric mucosa of rats from the different groups, while the quantitative analysis of the results of the effects of BWA-AI, BWA-tablet and BWA-suspension, on gastric ulcer index and mucus content in the gastric mucosa of rats with ethanol-induced ulcer are shown in Table 1. Oral administration of ethanol induced the formation of gastric lesions and reduced the mucus content in the animals of the positive control group with respect to the negative control group (healthy animals). BWA-AI significantly reduced gastric ulcer index only at the dose of 200 mg/kg, reaching a percentage of inhibition close to 50 %, while the lowest dose assayed of 25 mg/kg did not prevent gastric lesions. However, both BWA-tablet and BWA-suspension treatments at 25 and 200 mg/kg significantly reduced the gastric ulcer index compared to positive control group (with damage), as well as respect to BWA-AI- treated group at the same doses. Treatment with BWA-suspension (93.9 and 99.8 % of inhibitions) was also more effective than BWA-tablets (37.0 and 75.4 % of inhibitions) for inhibiting the gastric ulcer index, at doses of 25 and 200 mg/kg, respectively.
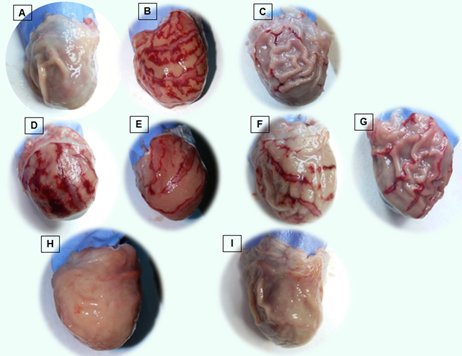
Figure. 1 Macroscopic examination of gastric mucosa of stomach of rats in A: Negative control group, (B) Positive control group, (C): Omeprazole treated group, (D-I) treatment groups at doses 25 and 200 mg/kg, respectively: (D, E) BWA-AI, (F, G) BWA-tablet, (H, I) BWA-suspension.
All treatments, BWA-AI, BWA-tablet and BWA-suspension, increased the mucus content of the gastric mucosa compared to the positive control group, without differences between them, and achieving total restoration (100%). OMP (reference substance) significantly prevented ulcers induction and increased mucus.
Furthermore, the animals of the positive control group (with damage) reached high levels of MDA and a decrease in the total proteins in mucus with respect to the basal levels observed in the negative control group (healthy animals), while all treatments significantly decreased MDA levels and increased the amount of total proteins (Table 2).
Table 1 Effect of oral administration of BWA-AI, BWA-tablet and BWA-suspension, on lesion size and mucus content in gastric mucosa of rats with ethanol-induced ulcer.
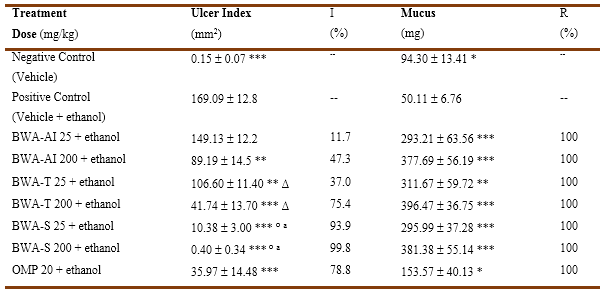
BWA-AI: BWA-active ingredient, BWA-T: BWA-tablet, BWA-S: BWA-suspension, OMP: omeprazole, R: restoration, I: inhibition. Data as Mean ( SEM (standard error of the mean) * p (0.05, ** p (0.01, *** p (0.0001 comparison vs positive control, ∆ p( 0.05, ° p (0.0001 comparison vs similar dose of BWA-AI, a p (0.0001 comparison vs similar doses of BWA-tablet (Mann Whitney U test)
Table 2 Effect of oral administration of BWA-AI, BWA-tablet and BWA-suspension on malondialdehyde and total proteins in gastric mucosa of rats with ethanol-induced ulcer.
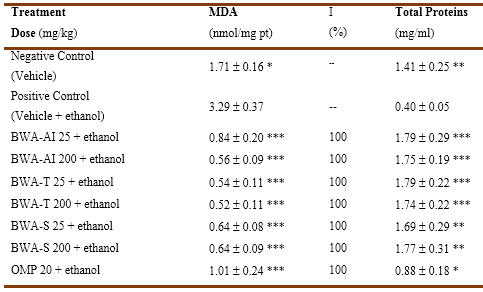
I: inhibition, BWA-AI: BWA-active ingredient, BWA-T: BWA-tablet, BWA-S: BWA-suspension, OMP: omeprazole, MDA: malondialdehyde. Data as Mean ( SEM (standard error of the mean) * p(0.01, ** p (0.001, *** p (0.00001 comparison vs positive control (Mann Whitney U test)
DISCUSSION
The results of this study corroborate the protective effects of BWA-AI and BWA-tablet, on the gastric mucosa, while demonstrating that the new BWA-suspension made from the active ingredient of the Abexol® tablet exerts the same pharmacological effects.
Gastric ulcer experimentally induced by ethanol in rats is characterized by acute erosive lesions, hemorrhagic areas accompanied by edema and necrosis (Zambrano and Roger, 2021; Ermis et al. 2023; Stern, 2023), as could be observed macroscopically in the stomachs of the rats of the positive control group, past one hour of the single oral administration of 60 % ethanol, unlike the negative control group (without damage) that showed the normal structure of the gastric tissue. The single oral administration of BWA-tablet and BWA-suspension decreased the formation of gastric ulcers and hemorrhagic areas with greater preservation of normal tissue from the lowest dose tested (25 mg/kg), while BWA-AI prevented damage to the gastric mucosa only at the mayor dose evaluated.
It is noteworthy that the BWA-suspension protected the gastric mucosa with a major efficacy than BWA-AI and the BWA-tablet, achieving a 99 % of inhibition, higher than those achieved by these two treatments (47 and 75 % of inhibition, respectively). On the other hand, BWA-suspension and BWA-tablet were more potent than BWA-AI, since they were effective from the lowest dose tested of 25 mg/kg, unlike the latter which was only effective with 200 mg/kg; being even BWA-suspension the most potent since it achieved the higher efficacy with the lower doses assayed.
Furthermore, all treated groups (BWA-AI, BWA-tablet and BWA-suspension) showed an increase in gastric mucus content, even reaching values above baseline (negative control group, without damage), which corresponds to the antecedents that support the efficacy of BWA-AI, through a mechanism that involves the increase of quantity of the mucus (Molina et al. 2001; Pérez et al. 2012, 2013; Carbajal et al. 1995; Molina et al. 2012; Carbajal et al. 1996; Molina et al. 2005; Carbajal et al. 2000).
The superior effect of BWA-suspension could be explained by the pharmaceutical form, since the suspension has greater bioavailability, in addition to the fact that microcrystalline cellulose is among the ingredients of the suspension, which has a proven anti-ulcer effect (Barzaga et al. 2004). Also, the OMP-reference substance also decreased the index of ulcers, which corroborates the validity of the results obtained in our experimental conditions (Molina et al. 2017).
Based on the fact that the induction of gastric ulcer by oral administration of ethanol to rats involves several mechanisms, including the depletion of endogenous prostaglandins that constitute mucosal defense factors and the increased levels of thromboxane A2 (TxA2), as well as a decrease in the quantity and quality of gastric mucus together with an increase in the activity of the enzyme myeloperoxidase (MPO), (Carbajal et al. 1995; Carbajal et al. 1996; Carbajal et al. 2000; Molina et al. 2015) the anti-ulcer effects of the evaluated treatments could be related to the previously demonstrated ability of the active substance (BWA-AI) to restore mucus content and PgE2 levels in the gastric mucosa, decrease neutrophil infiltration and inhibit the activity of MPO, COX-2 and 5-LOX (Pérez et al. 2012, 2013; Molina et al. 2017; Pérez et al. 2015).
On the other hand, ethanol produced an increase in MDA concentrations and protein depletion in the gastric mucosa, while oral treatment with BWA-AI, BWA-tablet and BWA-suspension at the doses tested produced a marked and significant effect on both variables.
The effect achieved with the evaluated treatments (BWA-AI, BWA-tablet and BWA-suspension) was 100 % inhibition on MDA production and an increase in protein concentrations above baseline.
These results corroborate the efficacy of BWA-AI on gastric ulcer and ethanol-induced oxidative stress while demonstrating that the formulations BWA-tablet and BWA-suspension exert a superior effect than BWA-AI on gastric ulcer, an effect that in turn was superior with BWA-suspension.














