Introduction
Dengue virus (DENV) infection causes either a feverish self-limited disease (often including rash and body pains) or a severe syndrome that is characterized by systemic capillary leakage, bleeding with or without thrombocytopenia and hypovolemic shock. Severity is mainly due to secondary infection by a heterotypic DENV1 and is now considered a global threat.2,3 The number of severe dengue (SD) patients requiring admission in Intensive Care Units remains high4 and any piece of information that could help to distinguish patients with SD with a higher risk of death becomes crucial.5 The World Health Organization (WHO) 2009 dengue case classification (WHO/DCC-2009) includes three categories: Dengue without Warning Signs (D), Dengue with WS (D+WS) and SD. It also recognizes the existence of a continuous spectrum of dengue disease.6 This was validated in multicenter studies,7,8 recommended to be utilized by Pan American Health Organization (PAHO)9 and included in the Guidelines for Dengue Management in all the Countries in the American Region.10,11,12 A retrospective study of risk factors and predictors of severe dengue in particular populations has been made13,14 and a systematic review and meta-analysis of risk predictors of progression to severe disease during the febrile phase of dengue.15 We describe the main clinical characteristics and outcomes of adult dengue patients admitted at the Institute of Tropical Medicine Pedro Kourí (IPK) in Havana, Cuba, during three dengue epidemics to analyze the usefulness of WS and challenges of the WHO/DCC-2009 in preventing and managing severe dengue cases.
Methods
Ethics statement
The Clinical Research Ethical Committee of IPK approved the study. All data were anonymized.
Patients
During the 2001-2002, 2006 and 2012 dengue epidemic, all adult patients with confirmed diagnosis of dengue were admitted to the IPK hospital and were included in the present study. In 2001-2002, IPK admitted patients with Dengue or SD, who were transferred from all hospital in Havana City. In 2006 and 2012, the patients with WS were admitted not only at the IPK hospital (the National Reference Centre for Infectious Diseases in Cuba) but also to other Hospitals in Havana. They were managed using a standardized dengue clinical-care protocol. During the first two periods (2001-02 and 2006), the clinical management of patients was conducted according to the PAHO 1995 guidelines,16 which included a group of WS along with their commended early fluid replacement regime for those cases, not excluding SD. In 2012, patients were managed according to WHO guidelines edited in 2009,6 and with its version for the American Region: PAHO 2010 guidelines.17 During the epidemic of 2001-02 the medical team has received prospective clinical training. Data was obtained retrospectively from medical records, extracting demographic, clinical, laboratory, radiological, treatment and outcome data of all laboratory-confirmed dengue-inpatients in 2001-2002 epidemic. During 2006 and 2012, both training and obtaining clinical data were prospective. Polymerase Chain Reaction (PCR) obtained confirmation and identification of infecting serotype and the information about circulating serotypes was taken from surveillance data of the National Reference Laboratory.
Clinical outcome
Patients were diagnosed with dengue haemorrhagic fever (DHF), according to the WHO/DCC-1997, if they had fever, thrombocytopenia ((100,000 x mm3), any bleeding, some evidence of plasma leakage (haematocrit ≥20%, or clinical fluid accumulation) and with dengue shock syndrome (DSS) when shock was also present.18 When applying WHO/DCC-2009, cases with evidence of plasma leakage associated with shock or respiratory distress (defined as the American-European Consensus Conference,19 were considered SD as well as those cases with severe bleeding and/or severe organ involvement.
Warning signs
WS recorded included: intense and continuous abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation (pleural effusion or ascites detected by physical or radiological examination), mucosal bleeding, lethargy / restlessness, hepatomegaly, and an increase in haematocrit to be considered as WS with any of the described parameters for WS should be present at the beginning or during the so-called critical phase (24 hours before defervescence or 48 hours later). In PAHO 2010 guidelines, other symptoms such as altered level of consciousness, related with hypotension due to plasma leakage: lipothymia and somnolence;17 were included as WS.
Data analysis
For describing cases (including fatalities), comparing the WHO/DCC-2009 to the previous WHO/DCC-1997 and determining the performance of WS to predict SD (specificity, sensitivity, negative and positive predictive values) we evaluated all patients (years: 2001-2012).
With prospectively obtained data (2006-2012), we analyzed how long each WS have preceded shock and others symptoms associated to shock.
For the evaluation of the intravenous fluid treatment (IV-fluid-treatment) impact all the history charts of each patient of 2012 epidemic were analyzed. Every patient classified as D+WS and SD according to the WHO/DCC-2009 classification were included.
The patients with severe bleeding and organ impairment without shock were excluded having considered that WS are a clinical expression of plasma leakage, not other form of dengue severity.
To classify a given treatment as correct or not, the time elapsed from the beginning of WS and the initiation of the IV fluid was considered: if the IV-fluid-Treatment was initiated 12 hours or later after the WS were identified, it was considered as incorrect. The adequate doses of crystalloids was 10 mL/kg in one hour, according by PAHO guidelines.17 To measure the impact of treatment, we calculated the proportion of patient with correct treatment and had a good outcome, not developing SD.
Descriptive analyses, frequency and percentages were used for categorical variables. The continuous variables, means and standard deviations were used; also along with the statistical package SPSS v. 19.
Results
Dengue cases (1439 adult dengue inpatient) included in this study are shown in Table 1 according to both classifications. In the epidemic of 2001-02 (921 patients) only DENV serotype 3 (DENV-3) was confirmed.20 DENV-4 was identified in 2006 (271 patients), and DENV-3 and DENV- in 2012 (247). The median age was 37 years (range: 19-79 years), 766 (53.2%) were female and 1053 (73.2%) had white coloured skin.
Outcomes
In terms of clinical outcomes, using WHO/DCC-1997, 1092 patients had DF and 347 had DHF. According to the WHO/DCC-2009, 287 had D, 1109 had D+WS and 43 were cases with SD. 896 of DF cases had at least one WS and 15 were classified as SD cases, and213 of DHF were later considered as D+WS cases; while 28 were SD (Table 1).
Table 1 Comparison of dengue cases (number and per cent; n [%]) classified by WHO/DCC-2009 and 1997
| WHO/DCC-1997 | WHO/DCC-2009 | Total | ||
| Dengue | Severe dengue | |||
| Without warning sign | With warning sign | |||
| DF | 181 (12.6) | 896 (62.3) | 15 (1.0) | 1092 (75.9) |
| DHF(DSS | 106 (7.4) | 213 (14.8) | 28 (1.9) | 347 (21.1) |
| TOTAL | 287 (19.9) | 1109 (77.1) | 43 (3.0) | 1439 (100.0) |
WHO, World Health Organization; DCC, dengue case classification; DF, dengue fever; DHF, dengue haemorrhagic fever; DSS, dengue shock syndrome.
Among 43 patients with SD, 41 (95.3%) had severe plasma-leakage with shock (none with respiratory distress, at least at the beginning of the severe clinical picture), one patient (2.3%) had severe bleeding (severe digestive bleeding not associated to plasma leakage) and one had severe organ involvement (2.3%) who had clinical signs of encephalopathy (Table 2). Shock was evident at day 4.9(1.1 (median(standard deviation).
Table 2 -Severe dengue cases, including criteria of severity
| Severe dengue (n [%]) | 2001-2002 | 2006 | 2012 | Total (%) |
| 19 (2.1) | 16 (5.9) | 8 (3.2) | 43 (3.0) | |
| Shock (plasma leakage) | 19 (100.0) | 16 (100.0) | 6 (75.0) | 41 (95.3) |
| Severe bleeding | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (2.3) |
| Severe organ impairment | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (2.3) |
| Severe hepatic injury | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Myocarditis/Myocardiopathy | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Encephalitis/Encephalopathy | 0 (0.0) | 0 (0.0) | 1 (100.0) | - |
Warning signs
WS identified at the first presentation to hospital and at the day of final clinical outcome (SD or Non-SD) are shown in Table 3. One to three WS were present in the same patient mostly per case. The most common WS was mucosal bleeding: 926 (64.4%). This WS was very prominent during the epidemic 2001-2002 (806, 87.5%), since at that time any bleeding triggered admission to the hospital at IPK related at least partially to the criteria of severity due to bleeding, which was rooted in the clinicians and because the classification for severe cases as DHF was mainly related to them. When WHO/DDC 2009 classification was introduced, those concepts were modified and other WS became important for admission to the hospital. Frequent WS were abdominal pain or tenderness, 404 (35.5%) and frequent vomiting, 361 (25.1%). Clinical fluid accumulation in body cavities was considered as a WS in 66 patients (4.3%). Some symptoms associated with altered level of consciousness were also considered as WS: lethargy/restlessness or somnolence that were recorded in 18 patients (1.9%) and lipothymia (fainting or orthostatic hypotension) in 87 (9.4%). Prostration was a frequent symptom: 408 cases (28.4%).
Table 3 -Occurrence of warning signs and other symptoms in patients admitted during epidemics
| Warning signs* and other symptoms in patients admitted during epidemics |
2001-2002 (921) n (%) |
2006 (271) n (%) |
2012 (247) n (%) |
Total (1439) n (%) |
| Mucosal bleeding* | 806 (87.5) | 81 (29.9) | 39 (15.8) | 926 (64.4) |
| Abdominal pain or tenderness* | 250 (27.1) | 89 (32.8) | 65 (23.6) | 404 (35.5) |
| Frequent vomiting* | 293 (31.8) | 46 (17.0) | 22 (8.9) | 361 (25.1) |
| Clinical fluid accumulation in body cavities* | 16 (1.7) | 35 (12.9) | 15 (6.1) | 66 (4.6) |
| Progressive haematocrit rise* | - | - | 42 (17.0) | 42 (17.0) |
| Lethargy/restlessness*, or somnolence | 5 (0.5) | 9 (3.3) | 4 (1.6) | 18 (1.3) |
| Hepatomegaly>2cm* | - | 7 (2.6) | 2 (0.8) | 9 (0.6) |
| Prostration | 301 (32.7) | 55 (20.3) | 52 (21.1) | 408 (28.4) |
| Lipothymia (fainting) | 13 (1.4) | 19 (7.0) | 55 (22.3) | 87 (6.0) |
Prediction of shock
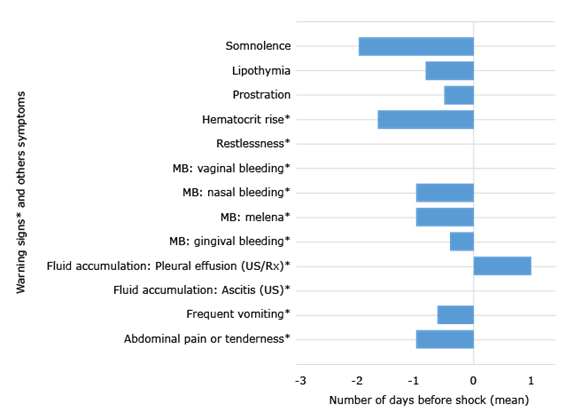
All symptoms, in relation with shock, had negative predictive values >95%. Sensitivity was >75% for mucosal bleeding, abdominal pain and tenderness. Specificity was >90% for lethargy/restlessness and lipothymia. Hepatomegaly was only observed in prospective studies: 2006 and 2012 dengue epidemic bursts, with 518 dengue cases as a baseline (Table 4). Most WS were identified 12-24 hours prior to shock (Fig.). Somnolence was evident up to two days in advance, as the haematocrit rise, but diagnosis of pleural effusion was made only after shock.
Table 4 - Performance of warning signs and selected symptoms to predict shock
| Warning signs* and selected symptoms | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| Mucosal bleeding* | 78.0 | 36.1 | 3.5 | 98.2 |
| Abdominal pain/tenderness* | 82.9 | 73.5 | 8.4 | 99.3 |
| Frequent vomiting* | 61.0 | 75.9 | 6.9 | 98.5 |
| Hepatomegaly >2cm*2006-2012) | - | 98.2 | - | 95.7 |
| Lethargy/restlessness* | 2.4 | 98.8 | 5.6 | 97.2 |
| Lipothymia | 34.15 | 94.8 | 16.1 | 98.0 |
Case fatality
Only one fatal case fulfilled SD criteria on admission day, when presented with two WS: abdominal pain and tenderness, and a rise in haematocrit value concurrent with platelet count drop. However, when applying WHO/DCC-1997 classification, DHF criteria were only met after two days after admission.
Treatment
The impact of IV-fluid-treatment was analysed prospectively in 139/ 247cases during the 2012 epidemic (133 D+WS, and six SD cases). We excluded two patients because capillary leakage was not the cause of severity: one with SD due to organ impairment and other with severe bleeding. The other 106 patients did not have WS and were not included in the analysis because intravenous fluid replacement was not necessary (Table 5). 133 patients with D+WS were managed with crystalloid solutions. Nine cases were not managed according to the guidelines, including the six SD patients above mentioned plus three cases with D+WS. The patients with incorrect treatment were those who for different reasons, arrived late at hospital health service. Thus, 100% of SD did not received the correct treatment (100%, 95% IC: 61.0-100%) and 97.7% (95% IC: 93.6-99.2%) of patients managed with the recommended treatment were not in shock).
Table 5 -Dengue cases and its treatment with crystalloid solutions during 2012 epidemic
| Dengue cases classified according to WHO/DCC-2009 classification (number of patient) | Patients treated with crystalloid solutions | |
| Total n (%) | Correct treatment n (%) | |
| Dengue (106) | 3 (2.8) | Not analyzed |
| Dengue with warning signs (133) | 133 (100.0) | 130 (97.7%) |
| Severe dengue due to plasma leakage (6) | 6 (100.0) | 0 (0%) |
Discussion
With no specific therapy available, effective case management of patients with dengue relies on frequent monitoring and judicious use of IV-fluid-treatment with isotonic crystalloids. WS help doctors to initiate this treatment at the right moment. In the current study, we found that on time and correct treatment (based on management of WS) avoided severity (100%).
This study also confirms the usefulness of the WHO/DCC-2009, with greater discriminatory powerful detecting patients at risk of progression to SD.21,22,23 It is simple to be applied by clinicians for triage and case management -according to disease severity- even in primary care settings24,25and disease surveillance. It also reflects the natural course from mild to severe disease and covers all clinical manifestations.26 A formal expert consensus on its usefulness was reached in Havana, Cuba27 with dengue experts from the Americas sharing experiences on the application of it. All countries in the Americas have incorporated the WHO/DCC-2009 in their national guidelines for dengue. A decrease in case fatality has been documented in the Americas,28 after capacity building programmes were introduced,29 in combination with emergency planning for dengue outbreaks,30 including reorganisation of the health care facility response.31 As the current study documents, applying those guidelines only one case fatality occurred, who presented late at the hospital already fulfilling SD criteria on admission day, dying of severe bleeding.
Shock occurs when a critical volume of plasma is lost through leakage.6 WS precede symptoms of clinical shock. Some studies are identifying which of the WS are more frequent and have more significance to predict severity.32,33 Studies in Asia have shown that bleeding manifestations are more common in adults than in children.34 In our study, haemorrhages were rare as a cause of SD, but mucosal bleedings were the most frequent WS (926, 64.4%) because they were well differentiated from other haemorrhages, mainly from the digestive tract or lungs. Other clinical researchers have included “any gastrointestinal” bleeding, any “internal” bleeding (retroperitoneal or intracranial), menorrhagia or inter-menstrual bleeding (not controlled by progesterone), or any clinical bleeding requiring endoscopy or surgery.35 Obviously, criteria of severe bleeding versus mucosal bleeding continue being a challenge.
Abdominal pain or tenderness in 404 patients (35.5%) and frequent vomiting were important WS: both appeared before the day of shock, with a sensitivity of 82.3% for the former and 61.0% for the latter predicting severity. Abdominal pain has been related with severity in different studies: both in adults36,37 and children.38,39 Prostration was important in SD cases; this symptom was frequent (71.9%) in 121 dengue fatalities during epidemics in the State of Ceará, Brazil.40 These studies analysing dengue case fatalities show that 100% (121/121) of the cases were classified as SD (WHO/DCC-2009), while only a relative small number of cases fulfilled DHF/DSS criteria.40 Somnolence was described up to two days before the onset of shock, but lethargy/restlessness appeared during shock. Branco et al. describe that epistaxis and persistent vomiting were strongly associated to death;5 both are considered as WS in the WHO/DCC-2009.6 No significant association are observed with any of the laboratory findings and death.5 In our study, fluid accumulation was only diagnosed after the onset of shock because it was not possible to do continuous surveillance of pleural effusion with Chest-X-Rays or of fluid collection on ultrasound.
The improved sensitivity of the WHO/DCC-2009 allows for better severe case detection and this may increase Intensive Care Unit admissions,41 but this may also avoid deaths due to SD. Clinical researchers in Asia developed predictive tools of higher risk of SD compatible with well-resourced and resource-limited settings, and not requiring laboratory measurements. These tools have included some of the WS proposed by WHO/DCC-2009: abdominal pain or tenderness and vomiting as predictors of combined forms of SD.42 In children, abdominal pain and vomiting and also ascites, pleural effusion and hepatomegaly, were found significantly higher in SD cases.43
WS do not necessarily apply to other forms of SD (haemorrhages or organ dysfunction severe). In some studies WS were correlated with these forms of SD,23 and this may be one explanation for the low positive predictive values of WS reported in those studies. Successful treatment of cases having WS may lead to underestimate the amount of SD cases, as occurred in a cohort of inpatients in Singapore.23
Rosenberger et al. in a study performed in hospitals in endemic countries from South-East Asia and Latin America found that the risk for respiratory distress with fluid accumulation increased significantly as the infused volume over the preceding 24h increased (hazard ratio 1.18 per 10 mL/kg increase; P<0.001). Longer duration of intravenous therapy, use of a fluid bolus in the preceding 24 h, female gender and poor nutrition also constituted independent risk factors, in patients with significant vascular leakage. They concluded that IV-fluid-Treatment may aggravate fluid accumulation and result in respiratory distress.44 In this study, 85% patients were Asian and 61.2% were children and adolescents (0-14 years). The results may be related to the subpopulations included. This is different to the results presented in our study in which no patients had respiratory distress, and IV-fluid-treatment with crystalloids was effective and safe.
The inclusion of severe organ impairment in the WHO/DCC-2009 as SD cases give clinicians the opportunity to report dengue cases that were previously ignored and help to describe the full extent of severe syndromes associated with dengue infection. However, some clinical researchers have considered that while the WHO/DCC-2009 is clinically useful, definitions of severe bleeding and organ impairment should be refined to improve clinical relevance and criteria for plasma leakage and hemodynamic compromise from WHO/DCC-1997 should be retained.41 It is a challenge for the definition of SD to better describe dengue myocarditis45,46,47,48 and other severe organ involvement: liver,49 kidney50,51 and others. New definitions of dengue encephalitis and dengue encephalopathy have been raised52 which need to be documented in clinical practice, because dengue affecting the central nervous system is progressively better recognised.
Limitations include that analysed information in this study was taken from different outbreaks, with the inherent implications for data quality. In 2001-02 epidemic, the analysis was made using secondary data but specific instrument to collect information was elaborated for the others. The clinical data could not always be taken prospectively, as desired, but medical staff was always trained for identifying the main symptoms and signs of dengue, particularly WS and for initiating early fluid replacement as the treatment protocol has stated. Not all cases were identified by similar laboratory tests. Only DENV-3 and DENV-4 were the identified serotypes, however, these viruses were known to be the most frequent serotypes circulating in the Americas at the time when the data was collected, and no other arbovirus was circulating in the country at that time, nor was vaccination against Yellow Fever in the population done. We did not explore the factors associated with severity of the disease such as secondary infection, serotypes of dengue virus and others. Data of comorbidities of patients was documented and will be analysed in another report.
Conclusions
WS were useful to predict SD due to plasma leakage/shock. Mucosal bleeding and abdominal pain/tenderness were very frequent and both had a good sensitivity to predict shock, the later having a better performance (specificity=94.5%). There were high probabilities of not to have shock in patients with absence of the evaluated symptoms (high negative predictive value). Lethargy / restlessness had good specificity to predict SD by shock, but not a good sensitivity. Lipothymia and prostration were symptoms that appeared in advance related to shock and with specificity to predict it >70%. The correct treatment with crystalloids in patients with D+WS avoided shock. The data also confirm that the WHO/DCC-2009 is clinically useful to manage dengue cases.