Introduction
The lectin pathway activates the complement system through the recognition of pathogens or altered-self-structures by mannan-binding lectin (MBL) or one of the three ficolins (H-, L- and M-ficolins) and collectin CL-LK.1 These lectin pathway starters can form complexes with serine proteases (MBL) like MASP-1, MASP-2, and MASP-3.2 Later, MASPs molecules are converted from pro-enzymes to active forms, leading to cleavage of C4 and C2; and generation of the C3 convertase.3
The function and diffusion of MASP-2 from blood into cerebrospinal fluid (CSF) was studied poorly.4 Nowadays, the knowledge of the nature of this molecule in this biological fluid is important firstly because of the role of the innate response in central nervous system and because it could be possible to explain the pathophysiology of many infectious and autoimmune disorders.1,2,3
Molecular diffusion/CSF flow theory5 is because the concentration of a blood-derived protein in cerebrospinal fluid (CSF) is in equilibrium between the rate of diffusion into CSF and the rate of elimination by CSF flow. Blood-CSF barrier and CSF flow rate increased protein concentration in CSF of patients with neurological disorders as blood-CSF barrier dysfunction due to pathologically reduced CSF flow rates.
The diffusion rate of the different molecules from blood to CSF depends on the molecular size and it is represented by CSF/serum concentration quotients of the blood-derived protein in normal CSF.
Albumin became the reference for the individual barrier function by the CSF/serum concentration quotient or Q Albumin. It is based because albumin in CSF is derived exclusively from blood in spite of the pathological processes of the neurological disorders.6,7
The diffusion rate for the passage for proteins from blood to CSF, the CSF/serum quotient of a blood-derived fraction of any protein molecule with a known molecular size can be calculated.7,8 According with this theory a change in Q Albumin can be interpreted of the pathologically reduced CSF flow rate. It is possible and necessary to extend these concepts to all proteins in CSF and the influence of reduced CSF flow rate on protein in CSF depends on the source of the proteins.
Sources of CSF proteins
Three sources for CSF proteins can be identified:
Blood-derived proteins in CSF 80% of total protein8 evaluated as CSF/serum quotients with reference to the albumin CSF/serum concentration quotient, Q Albumin (e.g. IgG, IgA, and IgM).8,9 The no linear reference range for blood-derived proteins in CSF forms the base for reibergrams,8 which enables the sensitive and quantitative detection of additional intrathecal synthesis.
Brain cell- derived proteins are interpreted by their absolute concentration in CSF without reference to Q Albumin, since they are independent of CSF flow rate like Tau protein and neuron-specific enolase.10,11
Proteins are released from leptomeningeal cells into CSF. These are also evaluated as absolute CSF concentration but with additional reference to Q Albumin, because they have a linear correlation with CSF flow rate (e.g. beta trace protein, MBL).12
Relatively new proteins like the ones that take part of the lectin pathway complement system shows different dynamics in CSF based on controls and patients, and can be supported by the molecular diffusion/CSF flow model of Reiber.8 From these considerations, it is possible to discriminate between the different sources of CSF proteins for diagnostic purposes. The results obtained in this paper will try to support the role of MASP-2 and its interaction with the lectin pathway starters following the dynamics from blood to CSF.
The scientific problem is to know the MASP-2 diffusion pattern from blood to cerebrospinal fluid and its relationship between soluble and associated forms.
The objective of this paper is to describe MASP-2 diffusion pattern from blood to cerebrospinal fluid.
Methods
A transversal observational prospective study was performed. CSF and serum samples were taken from patients of the Neurology Department, Göttingen University Hospital, Germany. All samples were taken for routine analysis,6,8,13) indicated by diagnostic criteria with the written informed consent of the patients. After routine analysis, residual CSF and serum samples were stored at -80°C anonymously, according to the ethics committee of Aarhus University laboratories.
From these residual CSF and serum samples, we selected retrospectively two groups for this study: 45 normal controls patients without organic brain disease with normal CSF and normal barrier function. Control patients were determined non-inflammatory disease to be normal according to clinical and imaging criteria, e.g. headache or non-inflammatory polyneuropathies, and according to their CSF and blood data (normal CSF leukocyte count and protein values), no oligoclonal IgG, age-related normal albumin quotient, normal blood leukocytes and serum C-reactive protein.
In addition, the second group with 11 patients with barrier dysfunctions without intrathecal immune response but with increased Q Albumin, i.e. with a blood/CSF barrier dysfunction. Patients with non-inflammatory diseases but with blood/CSF barrier dysfunction given for an increase of CSF/serum albumin quotients (Q Albumin) as well as all other blood-derived CSF/serum protein quotients (QIgG, QIgA, QIgM) but without any intrathecal synthesis of IgG, IgA and IgM. These patients did not have oligoclonal IgG in CSF. Patients had spinal canal stenosis, spinal tumor or disc prolapse.
All the samples had normal CSF cell counts and typical findings of these diseases in electromyography, magnetic resonance and tomography.
Cerebrospinal fluid was obtained by lumbar puncture and serum from blood was taken by venipuncture. Serum and CSF was stored frozen in aliquots at -80°C until analysis. Routine parameters such as albumin-, immunoglobulin- CSF/serum quotients, oligoclonal IgG, cell count, clinical and imaging criteria that were used to characterize the patient groups, were measured in the Neurochemistry Department of the University Hospital in Gottingen. CSF and serum albumin were quantified by immunochemical nephelometry with two-point or kinetic analysis by Wildemann y otros.14 Serum MASP-2 levels were measured by commercial enzyme-linked immunosorbent assay (ELISA) kit (Hycult Biotech, Uden, the Netherlands).14 CSFMASP-2 was quantify by the same method with undiluted CSF samples. MASP-2 diffusion rate was calculated by the mean of the individual QMASP-2 from controls. Expected MASP-2 was theoretically calculated by the following formula:
Equation 1
Expected CSF MASP-2= MASP-2 diffusion rate x Serum MASP-2
Equation 2
MASP-2 synthesized Intrathecally = CSF MASP-2 - Expected CSF MASP-2
Equation 3
Population coefficient variation (CV) = (Standard deviation / Arithmetic mean) x 100
Results
3.1 Protein concentrations
The complete set of protein data for CSF and serum of both groups (i.e., with and without a blood CSF barrier dysfunction) is shown in the table. The corresponding CSF/serum concentration quotients of MASP-2 (QMASP-2, Table 1) are shown as a function of the albumin quotient.
3.2 MASP-2 diffusion rate and expected CSF MASP-2
MASP-2 diffusion rate is the mean of the individual controls QMASP-2. Its value is 0.013. According to equation (MASP-2 synthesized Intrathecally = CSF MASP-2 - Expected CSF MASP-2) it is possible to say that 24 controls have more MASP-2 that it could be expected by simple diffusion from serum and it is possible to found in controls with normal Q albumin values like in controls with larger Q albumin values with barrier dysfunctions.
CSF/serum quotients for albumin and MASP-2 with individual CSF and serum concentrations of MASP-2 in controls and patients with barrier dysfunctions and the individual expected CSF MASP-2 and CSF MASP-2 can be found in Mendeley Data repository.15
The population coefficient of variation is a variable employed to explain the source of the protein found in CSF. If the CV value of a molecule is smaller in CSF of a group of patients than in the corresponding, serum of this group the molecule must derive primarily from brain. In case of blood-derived molecules in CSF, their CV in blood must be higher in CSF by the additional individual variation of CSF flow rate (Table).
Table- Descriptive statistic of variables
| Summary statistics | MASP-2 ng/L | MASP-2 CSF ng/L x 10-3 | Q MASP-2 | Q Albumin |
|---|---|---|---|---|
| Arithmetic mean | 326.71 | 3.56 | 0.0128 | 7.9929 |
| 95% CI | 271.5101 to 381.9185 | 2.6133 to 4.4982 | 0.0099 to 0.0157 | 5.4751 to 10.5106 |
| Standard deviation | 206.14 | 3.52 | 0.0108 | 9.4014 |
| CV% x 10-3 | 63.1 | 98.9 | - | - |
| Kolmogorov- Smirnov test | accept Normality (p = 0.122) | reject Normality (p < 0.001) | accept Normality (p = 0.204) | reject Normality (p < 0.001) |
n= 56; CV: population coefficient variation
In order to know the relation between the absolute values of MASP-2 in CSF with the variation of Q albumin as a control measure of diffusion rate from serum to CSF, correlation coefficient r was calculated. (r = 0.6762; p < 0.0001; 95% Confidence interval for r = 0.5026 to 0.7973) It means that an increased Q albumin produces a proportional increase of MASP-2. Their rostro-caudal concentration gradient is, as expected from the theory, linearly increasing.
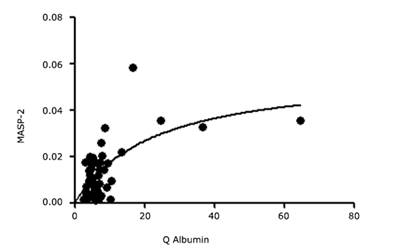
The correlation of the absolute CSF concentration of MASP-2 with the albumin CSF/serum quotient, Q Albumin, is shown in figure 1 for all patients without and with a barrier dysfunction (Table). This diagram shows graphically that MASP-2 concentration in lumbar CSF increased with increasing Q Albumin as correlation coefficient demonstrated.
Linear regression between CSF MASP-2 and Q Albumin indicate that the MASP-2 concentration in CSF increase with an increment of Q Albumin (MASP-2 = 1.5327 + 0.2531 (Q Albumin); p < 0.001).
This curve at figure 1 looks as a saturation function with increasing Q albumin. Taking into account the theoretical equilibrium between free CSF MASP-2 found and MASP-2 interacting subarachnoid environment, it could be explain more properly using the obtained QMASP-2.
On the other hand, there is also a significant statistic relationship between QMASP-2 and Q Albumin (MASP-2 = 0.0077 + 0.0006 (Q Albumin); p < 0.001), in a linear regression following the same behavior than the variables mentioned before when MASP-2 was employed.
In figure 2, it follows more properly the saturation curve found between QMASP-2 and Q Albumin.
Discussion
As other components of the lectin pathway the MASP-2 diffusion dynamics between blood to CSF remains unknown as well as its function in cerebrospinal fluid.
It is known that C3c16,17) and C4,16,17,18 as well as of other complement components like MBL19 were synthesized in CSF. It is possible to think that MASP-2 could be synthesized in this biological fluid as well as these other components.
According to the theory of the molecular diffusion/CSF flow model,8,16 it is possible to know the source of a protein found in CSF, i.e. if it is derived from blood, from brain or from leptomeninges. In addition, if the protein is interacting with the subarachnoid environment or part of and it could be found in a not soluble form.
MASP-2 concentration in CSF is lower than in serum. It indicates that MASP-2 is a blood-derived protein. It is well known that MASP-2 is synthesized by hepatocytes but it does not mean that it could be produced by other sources.16
Also, the brain-derived proteins, which are released into ventricular and cisternal CSF, are not modified by CSF flow rate. The results indicate that the concentration of MASP-2 depends on Q Albumin, so it was modified by CSF flow rate.
Both arguments support the fact that MASP-2 are not a brain-derived protein as Tau protein,20 neuron-specific enolase21 and other ones.
According the Reiber’s postulates about CSF low rate and source-related dynamics of the proteins from blood to CSF, the other condition that a molecule have to fulfill to consider or not derived primarily from brain is that the CV value should be smaller in CSF than the corresponding serum.
The biological coefficient of variation of a blood-derived protein in CSF in a group with severely increased albumin quotient, Q Albumin, is the same as in a group with a normal albumin quotient.22
The biological coefficient of variation of a blood-derived protein in CSF does not approach the value of the biological CV in the blood of the same group of patients.
When Reiber in 1994 studied it in more than 4300 controls pairs of CSF and serum in spite of the most severe barrier dysfunction with CSF protein concentrations approaching the concentration in serum.23
The biological variability, expressed as the coefficient of variation, for MASP-2 in CSF is higher (CV= 55%) than the serum MASP-2 value (CV= 40%). In the case of a blood-derived protein in CSF, an increased coefficient of variation would be expected, due to the additional biological variation originating from the individual barrier function.10,11) Therefore, MASP-2 in CSF is primarily a blood-derived protein but it is possible to be synthesized in the central nervous system (Table).
It is well known that MASP-2 have to interact with lectin pathway starters as MBL, ficolins and CL-LK24,25 and it is possible to find in a free form and linked with the lectin pathway complement starters.
The dynamics of MASP-2 from blood to CSF acts as a saturation function with increasing Q Albumin, as it is found for soluble intercellular adhesion molecule type 1 (s-ICAM-1)4,26. Soluble MASP-2 found in CSF is interacting with MASP-2 linked to the starters are not solely diffusion dependent.
ICAM-1, a cell surface receptor important for cellular interactions in immune responses, especially leukocyte trafficking into inflamed tissue; it is released in a soluble form (sICAM-1) into the extracellular space. About 60% to 80% of sICAM-1 in normal lumbar CSF derives from blood. This calculation is based on the theoretically expected molecular size-dependent blood-CSF gradient between 300:1 to 250:1.27
The experimental data indicates that the increment of MASP-2 in serum should not correspond with the expected CSF concentration. This paradox is unique for the proteins that follows a saturation behavioral and it is quite different from other proteins blood-derived like immunoglobulins and other complement components.12 That means that part of MASP-2 from blood to CSF was linked to MBL or other lectin pathways starters and it is the reason why is not possible to found the expected increment in CSF with the flow rate.
The limitations of the present study are due to the small number of samples included. Perhaps, a larger sample it is possible to underline with more precision these results. Nevertheless, up to the present it looks like that, MASP-2 is a blood-derived protein but it is possible to be synthesized in CSF as the other components of the lectin pathway already known.28,29
Conclusions
MASP-2 in CSF is predominantly blood-derived according at its concentration in CSF. The saturation curve demonstrates that MASP-2 interacts with the starters of the lectin pathway like MBL, ficolins and CL-LK. The dynamics of the diffusion from blood to CSF indicate a saturation curve where MASP-2 interacts with the subarachnoid environment. Nevertheless, an increment of the sample size should be better to improve these results.