Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.30 no.3 La Habana jul.-set. 2013
TECHNIQUE
Procedure for the conjugation of the Streptococcus pneumoniae serotype 6B capsular polysaccharide to the tetanus toxoid
Procedimiento de conjugación del polisacárido capsular de Streptococcus pneumoniae serotipo 6B a toxoide tetánico
Jean P Soubal1, Luis Peña1, Darielys Santana1, Yury Valdés1, Dagmar García2, Jessy Pedroso3, Félix Cardoso3, Humberto González4, Violeta Fernández5†, Vicente Vérez6
1 Departamento de Glicoconjugación. Centro de Química Biomolecular, CQB. Ave. 21 y Calle 200, Reparto Atabey, Apdo. 16042, Municipio Playa, CP 11600, La Habana, Cuba.
2 Departamento de Inmunología. Centro de Química Biomolecular, CQB. La Habana, Cuba.
3 Departamento de Análisis. Centro de Química Biomolecular, CQB. La Habana, Cuba.
4 Departamento de Desarrollo y Escalado, Instituto Finlay. Calle 27, No. 19805, La Lisa, AP 16017, CP 11600, La Habana, Cuba.
5 Dirección de Investigaciones. Centro de Química Biomolecular, CQB. La Habana, Cuba.
6 Dirección General. Centro de Química Biomolecular, CQB. La Habana, Cuba.
ABSTRACT
Streptococcus pneumoniae causes annually 826 000 deaths in children under five years. The serotype 6B, one of higher incidence, is targeted by the Cuban research and development project to develop a conjugate vaccine. There is limited data on how modifications caused by conjugation affect the physicochemical and antigenic characteristics of polysaccharides, particularly for serotype 6B capsular polysaccharide (PS6B), despite being the least immunogenic among S. pneumoniae polysaccharides. In this work, a conjugation procedure was established for PS6B comprising: fragmentation by acid hydrolysis, activation by periodate oxidation, and conjugation to tetanus toxoid (TT) by reductive amination to increase its immunogenicity. Reaction conditions were set to obtain the polysaccharide in three molecular size ranges (1-10, 10-30, 30-100 kDa) and levels of oxidation. PS6B fragmentation below 10 kDa and oxidation above 24 % of the repetitive units implied the loss of antigenicity. Polysaccharide length but not oxidation level had an impact on the physicochemical characteristics of the conjugates in the tested conditions. Unlike the native polysaccharide, conjugated 10-30 kDa and 30-100 kDa PS6B were immunogenic in rabbits, with evidence of thymus-dependent response. The procedure described supports obtaining PS6B-TT conjugates reproducibly in the 30-100 kDa and 10-30 kDa molecular size ranges and with 8-18 % oxidized repeat units, which are immunogenic.
Keywords: polysaccharide, conjugate, Streptococcus pneumoniae, 6B serotype.
RESUMEN
Anualmente Streptococcus pneumoniae provoca 826 mil muertes de niños menores de cinco años. El estudio del serotipo 6B, uno de los de mayor incidencia, es uno de los objetivos del proyecto de investigación-desarrollo para obtener una vacuna conjugada en Cuba. La estrategia de conjugación del polisacárido capsular del serotipo 6B (PS6B) fue fragmentarlo mediante hidrólisis ácida, activarlo mediante oxidación con peryodato y conjugarlo a toxoide tetánico (TT) mediante aminación-reductiva. Existe poca información sobre cómo estas modificaciones afectan las características físico-químicas y antigénicas del polisacárido, en particular para PS6B, a pesar de ser el menos inmunogénico de los polisacáridos de S. pneumoniae. En tal sentido, se estableció un procedimiento para obtener conjugados inmunogénicos de PS6B a TT. Se crearon condiciones de reacción para obtener el polisacárido en tres rangos de tallas y niveles de oxidación. Se determinó que la fragmentación del polisacárido por debajo de 10 kDa y la oxidación de más del 24 % de las unidades repetitivas implican pérdida de antigenicidad. La talla del polisacárido tuvo impacto en las características físico-químicas de los conjugados en las condiciones evaluadas; no así el nivel de oxidación. A diferencia del polisacárido nativo, conjugados de PS6B de 10 a 30 kDa y 30 a 100 kDa fueron inmunogénicos en conejos, con evidencias de respuesta timo-dependiente. Los procedimientos que incluyen la obtención del PS6B de 10 a 30 kDa y 30 a 100 kDa con niveles de oxidación entre 8 y 18 % de las unidades repetitivas oxidadas, permitieron obtener conjugados a TT reproducibles e inmunogénicos.
Palabras clave: polisacárido, conjugado, Streptococcus pneumoniae, serotipo 6B.
INTRODUCTION
Yearly, Streptococcus pneumoniae (pneumococcus) causes 14.5 millions of pneumonia, bacteriemia and meningitis episodes worldwide, with 826 000 deaths among infants younger than five years [1]. Vaccines against this bacterium have been available for the last 30 years, mostly based on the envelope capsular polysaccharides (PS), and from up to 23 different circulating serotypes. However, those vaccines are not immunogenic in children younger than 2 years, the most susceptible population. This happens because their immune system is unable to generate the adequate immune response against T-independent antigens, as the bacterial polysaccharides [2]. This problem has been addressed by developing conjugate vaccines, capable of inducing the required T-dependent immune responses against PS [3]. The first of these vaccines was licensed in 2000 (Prevenar®, against seven serotypes), and more recently other two (Synflorix® and Prevenar 13®, against 10 and 13 serotypes, respectively), but at very high prices in the market. In Cuba, there is a project currently at the research-development phase to develop a pneumococcal conjugate vaccine covering seven serotypes on its first stage: 1, 5, 6B, 14, 18C, 19F and 23F.
Among them, serotype 6B is one of the most frequently causing invasive diseases in children younger than five years in countries either developed or underdeveloped [4], like in Cuba and its geographic area [5]. It is also one of the most common causes of otitis media worldwide [6]. The three commercially available vaccine formulations contain the serotype 6B PS (PS6B), a component of most of the vaccine candidates assayed in clinical trials. Most of the studies describe the conjugate of this serotype as the less immunogenic [7, 8]. Its repetitive unit (RU) comprises the sequence →2)-α-D-Galp-(1-3)-α-D-Glcp-(1-3)-α-L-Rhap-(1-4)-D-RibOH-(5-PO4→ [9]. This polymeric structure is linear, with phosphodiester bonds which confers it a periodic negative charge. Its low immunogenicity results from its relatively simple structure and its similarity to DNA [10].
The conjugation strategy proposed by our project comprises the fragmentation of PS by acid hydrolysis, followed by PS activation by periodate oxidation and conjugation to the tetanus toxoid (TT) by reductive amination. Although this conjugation method is frequently used, there are few studies elucidating how such modifications affect the physicochemical and antigenic properties of PS in general. Particularly for PS6B, it has not been previously studied, in spite of its low immunogenicity. PS modifications during the conjugation process could affect the natural epitope conformation and promote the appearance of neoantigenic structures that subsequently affect the specificity of the immune response attained [11]. Therefore, the present study established a procedure to conjugate the PS6B to TT. It required to establish reproducible reaction conditions on each step and to determine the size and activation levels required for PS in order to preserve its epitopes and to efficiently generate its immunogenic conjugates.
MATERIALS AND METHODS
PS from serotypes 6B (batch 806), 18C (batch 801) and 19F (batch 802), and TT protein (batch 6017), were produced by the Development and Scale up department, and the Production plant II, respectively of the Finlay Institute, Havana, Cuba.
Fragmentation of native PS6B
The dissolution of 50 mg of PS6B was added with either a dissolution of acetic acid (Merck) or trifluoroacetic acid (TFA; Merck, Germany) to a final concentration of 0.25 M or 0.1 M, respectively. Each dissolution was further incubated at 70 °C and aliquots were collected at different times (Table 1). Aliquots were neutralized and molecular size fragmented by ultrafiltration (Amicon, Millipore Corp., Bedford, MA), with distilled water using regenerated cellulose membranes (Millipore, USA) with subsequent molecular cutoffs of 100, 30, 10 and 1 kDa. Each fraction was denominated according to the interval of cutoff values of the membrane retaining it and the one of the previous fragmentation step, and carbohydrate content was determined. The condition providing the highest yield for each fraction was reproduced, and the products obtained were analyzed attending to carbohydrate content, by monodimensional proton nuclear magnetic resonance (NMR-1H), size exclusion-high performance liquid chromatography (SE-HPLC) and in antigenicity studies. Yields (Y) were determined according to the following formula:
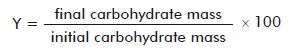
Activation of fragmented PS6B
A dissolution of 20 mg of fragmented PS6B (fractions 10-30 kDa or 30-100 kDa) was added with a sodium periodate dissolution (NaIO4; Riedel-de Haen) to final concentrations of 2.5, 5 or 10 mM in PBS, pH 7.0, and were further incubated for 3 h in the darkness. The reaction was stopped by adding 5 µL of glycerol (Plusone). The reaction mix was diafiltered against 5 volumes of distilled water, using a membrane with cutoff values of 10 kDa for the 10-30 kDa fraction or 30 kDa for the 30-100 kDa fraction. The products obtained were analyzed for carbohydrate and aldehyde groups content, by NMR-1H, SE-HPLC and antigenicity. The activation level was expressed as the percentage of oxidized repetitive units (ORU), calculated by the following formula:

where: RU: repetitive units
Yield was determined ad in the fragmentation study.
Conjugation of activated PS6B
Polysaccharides from fractions 10-30 kDa and 30-100 kDa were used, with ORU percentage ranges 8-12 and 13-18%. PS (20 mg) were added with 1 mL of 10 mg/mL TT dissolution. Subsequently, 4 mg of sodium borohydride (Merck, Germany) were added and incubated for approximately 18 h at room temperature. Two milligrams of sodium borohydride were further added and incubated for 2 h to eliminate the remaining aldehyde groups. The reaction mix was diafiltered against 10 volumes of distilled water with a 100 kDa cutoff membrane. The products obtained were analyzed for carbohydrate and protein content, by NMR-1H, SE-HPLC and antigenicity.
Analytical methods
Carbohydrate content was determined by the orcinol-sulfuric acid method [12] using a glucose standard curve. Carbonyl group content was determined by the modified Park-Johnson’s method [13] against a glucose standard curve. Protein content of the conjugates was assessed by the Lowry’s method [14], with a bovine serum albumin (BSA) standard curve.
RU structure was followed by NMR-1H. The samples were prepared in D2O. Spectra were obtained at 25 °C using a 250 MHz equipment (Brüker AC-250F). Signals were initially assigned according to van Dam et al. [15]. Chromatography analyses were done by SE-HPLC (Knauer Smartline, Germany) using a TSK 5000PW (TOSOH) column with refractive index detector. The distribution constant (KD) was calculated as follows:
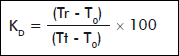
where:
Tr: sample retention time;
T0: retention time of a 2000 kDa dextran standard (Blue Dextran, Sigma);
Tt: NaN3 retention time.
The relative molecular weight (MWr) was determined by using a calibration curve of dextran standards (American Polymer Standard Corp.). The width at half peak height was determined with the program ClarityChrom 2.4.4.80 (Knauer, Germany).
The unbound protein content was estimated by the same SE-HPLC system using a Superose 12 column (Pharmacia, USA) with ultraviolet detector. The area under the curve for each peak was integrated with the ClarityChrom 2.4.4.80 program.
Antigenicity determination
The antigenicity of polysaccharide derivatives was determined by an inhibition immunoenzymatic assay (ELISA). A standard serum against SP6B (factor 6c, Statem Serum Institute, Copenhague, Denmark) diluted 1/3200 was used, followed by incubation with each inhibitor at concentrations ranging 0.005-500 µg/mL, overnight at 4 °C. The fragmented polysaccharides were used as inhibitors, either active or conjugated accordingly. Additionally, serotypes 18C and 19F PS were included as negative controls (non-related polysaccharides). For ELISA assays, polystyrene 96-well microtiter plates (Maxisorp, Nunc, Denmark) were coated with 10 µg/mL PS6B in phosphate buffer saline (PBS), pH 7.2, and incubated overnight at 37 °C. Plates were subsequently blocked with 1 % BSA for 30 min at 37 °C, and then, the anti-SP6B standard serum preincubated with the different inhibitors was added. Afterwards, the anti-rabbit IgG-horseradish peroxidase conjugate (HRP; Sigma, Germany) was added at a 1:10 000 dilution (in PBS, 0.3 % Tween-20, 0.01 M EDTA, 1 % BSA) and further incubated for 90 min at room temperature. Microplates were washed thrice after each step with washing solution (PBS, 0.05 % Tween-20). Finally, the HRP substrate solution was added (0.5 mg/mL ortho-phenylenediamine and 0.1 % (v/v) H2O2) in citrate buffer solution, pH 5.6, and the reaction was developed for 20 min in the darkness. The reaction was stopped by adding 3 M HCl. Absorbance was determined at 492 nm and the percent inhibition (PI) was calculated as follows:

Rabbit immunization
Two conjugates obtained from PS activated at ORU percentages ranging 13-18 % from fractions 10-30 kDa (Cj10-30) and 30-100 kDa (Cj30-100) were administered. Three groups of New Zealand White rabbits (Cenpalab, Havana, Cuba), five animals each, were immunized with 4 µg of conjugated PS, 25 µg of non-conjugated PS or placebo, in aluminum phosphate. Three doses of each immunogen were administered on days 0 (start of the study), 14 and 28 respectively. Blood was extracted from all the animals on days 7, 21 and 35. Sera were stored at -20 °C until use. The study was conducted in compliance with international guidelines for ethical conduct in the care and use of laboratory animals for scientific purposes. The Ethics Committee and the Quality Control Department of the Center of Biomolecular Chemistry (CQB, Havana, Cuba) approved all the experimental procedures.
Evaluation of the immune response induced by the conjugates
The IgG antibody response against PS was determined by an indirect ELISA, using the native PS6B for coating [16]. The antibody titer was calculated by a regression analysis between optical density (OD) values and the base ten log of the reciprocal of the serum dilution. Antibody titer was considered as the reciprocal of the serum dilution at which the absorbance reached twice the value of the 1/100 pre-immune serum. Titers above 50 were regarded as positive.
The IgG response against the TT carrier protein was determined as previously described to assess the response against the polysaccharide. In this case, plates were coated with 1 µg/mL TT in PBS, pH 7.2.
IgG antibody avidity against PS6B was determined by an ELISA similar to that used for characterizing the response against the polysaccharide, with some modifications. After serum incubation, a step of incubation with 0.5 M ammonium thiocyanate (NH4SCN, Merck) for 15 min was applied to half of the replicates. The resulting two dilution curves for each sample, with and without NH4SCN allowed the calculation of the avidity index (AI) as follows [17]:

The serum dilution showing an absorbance value of half the value of the serum diluted 1/100 without NH4SCN was considered as titer.
Statistical analyses
Results of the physicochemical evaluations were expressed as arithmetic means ± standard deviation of five independent experiments (three in the case of conjugates); the variation coefficient was also calculated for fragmentation yields and PS oxidation levels. Immunological evaluations were expressed as arithmetic means ± standard deviation of five animals. The statistically significant differences between groups were determined by the non-parametric Kruskal-Wallis test, and when present, a Dunn’s test was used a posteriori. Means were compared against reference values by the Wilcoxon test. Probability values (p) lower than 0.05 were considered as statistically significant. All the analyses were done with the Graph Pad Prism 4.03 program.
RESULTS
Fragmentation of the capsular PS6B
PS was fragmented by acid hydrolysis. The study was carried out by two acids dissolutions, assayed at different times. The conditions yielding the highest PS amounts were: 2 h in 0.25 M acetic acid for the fraction 30-100 kDa, 1 h in 0.1 M TFA for the fraction 10-30 kDa, and 2 h in 0.1 M TFA for the fraction 1-10 kDa (Table 1). These conditions reproducibly yielded more than 50 % PS (Table 2). The three processes rendered variation coefficients (VC) below 6 %.
NMR-1H spectra recorded for the fragmented PS were very similar to that of the native PS, indicating that the RU structure was preserved. Low intensity signals at 3.22 ppm and around 2.1 ppm in the native PS spectrum corresponded to polysaccharide C [18]. This contaminant, common among pneumococcal PS, was found at very low amounts in the starting material and decreased even more after fragmentation. There were no signals of end-product monosacharides resulting from size reduction, not even in the 1-10 kDa PS fraction (Figure 1).
KD increased conversely with size reduction. W0.5 values for fragmented PS were lower than those of the native PS, indicating a decrease in polydispersion. The rMW for each fraction was higher than the cut-off values of the flat ultrafiltration membranes used (Table 2).
Antigenicity, evaluated by an inhibition ELISA and using a polyclonal serum specific for PS6B, was only similar to that of the native PS for fragmented PS fractions 30-100 kDa and 10-30 kDa. In fact, the concentrations of these two fractions required to achieve 50 % inhibition were in the same order of that of the native PS, while that of the PS fraction 1-10 kDa was two orders higher (Figure 2). For this reason, fractions 30-100 kDa and 10-30 kDa were selected for further experimentation.
Activation of fragmented PS6B
Fragmented PS were activated by periodate oxidation. A study was conducted for this reaction in fractions 10-30 kDa and 30-100 kDa, varying the NaIO4/PS ratio (Table 3). A linear relationship was achieved for the equivalent amounts of NaIO4 and the number of aldehyde groups generated (R2 = 1.0 in the 10-30 kDa fraction and R2 = 0.99 in the 30-100 kDa fraction). ORU VC obtained at the different conditions ranged 11-20 %. There were no differences between the results attained for both working fractions. More than 85 % of the starting amount of PS was recovered in all the experiments, indicating that the oxidation reaction conditions did not generate fragmentation.
NMR-1H spectra of activated PS showed the chemical shifts and relative intensities of signals typical of the non-inactivated PS fragments (Figure 3A). There were no signals corresponding to the carbonyl proton at lower fields. Noteworthy, two new signals appeared at 5.10 and 5.09 ppm following those of the anomeric protons of ramnose and glucose superimposed at 5.15 ppm. The relative intensities of those two new signals were higher for PS showing the highest oxidation level (Figure 3B). These correspond to the signal of the anomeric proton of ramnose produced by the shortage in ORU of ribitol.
KD values were the same as for the inactivated PS for the given fractions (Table 2 and Table 3). These results confirmed that none of the oxidation conditions used seems to generate PS fragmentation.
While evaluating antigenicity, PS with a similar activation level up to 17.2 % ORU behaved similar to the fragmented PS of the same size. For these PS, concentrations required for a 50 % inhibition of serum were 1 and 2 times that of the native PS, respectively. On the contrary, the PS activated at 24.4 % ORU showed a drop in the inhibition capacity depending on its concentration, requiring a concentration 25-fold than that of the native PS to achieve a 50 % inhibition. Activation at 62.5 % ORU caused a drastic decrease in the inhibition capacity (Figure 3B). These results indicate that the activated PS antigenicity becomes affected when ORU is higher than 24.4 %.
Conjugation of activated PS6B
Activated PS of the working fractions 10-30 kDa and 30-100 kDa were conjugated at two ranges of activation levels to TT. There was no free protein detected in the conjugates obtained. Figure 4A shows the typical chromatograms. The differences detected in the PS activation levels had no influence on the properties evaluated. Otherwise, PS from the 10-30 kDa had higher KD and lower PS/protein ratio than that obtained from the 30-100 kDa (Table 4).
NMR-1H of PS conjugates retained the typical chemical shifts and relative intensities. There were new signals of low intensities in the range 1.0-0.8 ppm, corresponding to the aliphatic amino acids of the protein (Figure 4B). On the other hand, the capacity to inhibit the reference serum remained almost the same. The concentration required to achieve 50 % inhibition were of the same order of magnitude compared to that of the activated PS prior to conjugation (Figure 4C). These results indicated that conjugation did not significantly affect either the structure or the exposure of PS.
Evaluation of the antibody immune response generated by the conjugates
The conjugates obtained from the fractions 10-30 kDa (Cj10-30) and 30-100 kDa (Cj30-100) were tested by subcutaneous immunization of New Zealand White rabbits in three doses. The non-conjugated PS was administered as control and did not induce positive anti-PS6B antibody titers at any of the time points evaluated, and the response remained at baseline level as placebo (Figure 5). On the contrary, both conjugates generated IgG antibody titers against PS, with logarithmic titers above 2 after the second dose. On day 21, the Cj30-100 conjugate antibody titer was very highly statistically significant compared to that of the Cj10-30 (p < 0.001). This difference did not remain after the third immunization, due to an increase in the immune response generated by Cj10-30 (p < 0.001); but not by Cj30-100 (Figure 5A).
The quality of the response was evaluated by determining the AI for antibodies against PS on days 21 and 35. After the second dose, sera from the group receiving Cj10-30 overcame the 50 % of inhibitory activity (IA), in contrast to those of the group immunized with Cj30-100, which were lower (p < 0.001). Following the third dose, IA increased in both groups (p < 0.001) to near 90 %, reaching a level similar to that of the reference serum (Figure 5B).
Regarding the response against the carrier protein, there were no differences after three doses among the groups immunized with both conjugates (p > 0.05) (Figure 5C); in spite of the TT dose being 70 % higher in the Cj10-30.
DISCUSSION
The methods available to obtain PS-protein conjugates are quite varied [19]; but few are suitable for vaccine development. The controlled periodate oxidation of PS was selected, followed by reductive amination of the produced aldehyde groups by the amino groups of the protein. This is a simple and scalable method, applicable to PS from pneumococcal serotypes included in the Cuban vaccine project. It is also the method used to produce the Prevenar® and Prevenar13® vaccines [20]. In contrast to procedures used to generate commercial vaccines against pneumococci, PS was fragmented and the desired size range selected. By these means, a more efficient and consistent process is guaranteed, also favoring the quality of the immune response generated while administering the conjugate [21]. Moreover, the difference in the rMW distribution of the conjugate supported the exclusion of part of the free PS, as indicated by the change in the PS/protein ratio of the final conjugates compared to the reaction mix. A significant amount of free PS for this serotype could decrease the conjugate immunogenicity, more significantly for PS at higher rMW [22]. Acid hydrolysis was selected among the PS fragmentation methods available, due to its applicability to most PS molecules of the vaccine candidate and the easiness for scale up.
A methodology was followed considering all the factors needed, even those counteracting, to find the most adequate reaction conditions. Particularly relevant were the preservation of PS natural epitopes and the enhancement of the thymus-dependent character of the antigen once conjugated, reaction times no longer than 3 h a day, highly reproducible and potentially robust processes, rendering PS yields above 50 %.
The fragmentation study involved two acids of different strengths. Acetic acid supported to obtain PS in the 30-100 kDa fraction and TFA in the fractions 10-30 kDa and 1-10 kDa. Yields were higher than predicted and the reproducibility was regarded as adequate. These conditions would require further adjustment when working with native PS batches of other rMW distributions.
The fragments retained the RU chemical structure in the three fractions, as expected regarding the absence of PS6B side chains susceptible to hydrolysis. Significantly, antigenicity was preserved at the higher size PS fractions, but was affected in the 1-10 kDa fraction. This evidenced the preservation of the conformational epitopes essential for recognizing the native PS which are absent at lower sizes. Although previous reports on this matter were not found in the literature for PS6B, there were previous studies with other PS, such as those from Streptococcus B type III [23, 24], Neisseria meningitidis serogroups Y and W135 [25] and S. pneumoniae serotype 14 [26, 27]. In this last PS, conformational epitopes were also relevant for the generation of functional antibodies by the conjugates [28].
Reproducible conditions for PS oxidation were established while studying the PS6B activation. A linear relation was found between the equivalent amount of NaIO4 and the number of aldehyde groups generated, which supported the generation of an activation level in a preselected narrow range.
The periodate oxydation reaction occurs between adjacent hydroxyl groups followed by the rupture of the bond between the involved carbons which become aldehydes. The PS6B RU bears three of such sites: between positions C1-C2 and C2-C3 in ribitol and C3-C4 in galactose. Following the reactivity order described by Kim et al. [29], the oxidation of this PS must occur completely in ribitol since it is a linear chain. In support of this affirmation, it is known that under oxidation conditions of 0.87 moles of NaIO4/mol of UR, the N. meningitidis serogroup W135 oxidation proceeds in the side chain of syalic acid at C7-C8 and C8-C9 positions, and not at the C2-C3 and C3-C4 of galactose [30].
In this work, the NMR-1H revealed signals congruent with the shielded anomeric proton of ramnose, due to a shortage of the adjacent ribitol residue. On the contrary, there was no signal for the unshielded anomeric proton of galactose, present when it is oxidized. This implies that no monosaccharide ring was broken, what should minimize any perturbation in the PS conformation. Nevertheless, there was a decrease in antigenicity for RU above 24 % in spite of the fact that no PS fragmentation was detected. This indicates that more than ¾ of ribitol residues were required intact for a proper antigenic recognition. Based on these results, the 0.71 moles of NaIO4/mol of RU oxidation conditions were avoided.
Conjugates obtained from PS of 8-12 % and 13-18 % ORU, and activated with 0.18 and 0.35 moles of NaIO4/mol of RU, respectively, showed no differences in any of the physicochemical parameters evaluated. Conjugate immunogenicity is not expected to vary when generated from PS of the same size without any other difference than in the activation level. It has been observed that PS conjugates of group B Streptococcus type III with higher 18-89 % ORU range, higher than the one evaluated by our group, showed increased immunogenicity due to a rise in conjugate cross-linking [31]. Besides, this result was not further reproduced, due to a slight decrease in immunogenicity at a higher ORU percentage [32].
Otherwise, the PS size did influence in the physicochemical properties of the conjugates, the 30-100 kDa fraction generating the highest rMW and PS/protein ratio. The characterization of conjugates generated from a similar size PS was highly reproducible.
The immune response induced by the conjugates was quantitatively and qualitatively higher than that obtained by the non-conjugated PS. The raise of IgG antibody titers and avidity after each dose of conjugate indicated a thymus-dependent response, involving affinity maturation [33]. The increase in antibody avidity tends to correlate with the increase in protective capacity [34].
The Cj30-100 was more immunogenic than Cj10-30 although no statistically significant after the third dose. This result coincides with that obtained by Daum et al. [35] in children immunized with oligosaccharide or polysacharide conjugate vaccines. These differences in the elicited immune responses could be influenced by the PS length, but also by the conjugate size [36], the PS/protein ratio [37] or a combination of both factors. In fact, several studies addressing the influence of PS length on the immunogenicity of their respective conjugates achieved different results [32, 38]. At the same time, the response induced by the Cj10-30 conjugate seems to be more thymus-dependent, due to a faster increase in avidity. The native structure of the polysaccharide determines its thymus-independent type 2 nature, and part of this characteristic was preserved once conjugated to a protein [39]; while conjugates of lower rMW could favor better antigen recognition, a T-dependent one, therefore maximizing the effect of the carrier protein [11]. Nevertheless, those differences after three doses for both conjugates generated IgG antibody responses similar in titers and avidity.
In summary, the procedures described herein, which included the fragmentation of PS6B at molecular size cut-offs of 10-30 and 30-100 kDa, respectively, and their activation at levels from 8 to 18 % of oxidized repetitive units, allowed to obtain reproducibly PS6B conjugates to TT which demonstrated to be immunogenic. The methodology followed to find and establish each reaction conditions is also applicable for establishing polysaccharide conjugation procedures for other pneumococcal serotypes and bacteria.
ACKNOWLEDGEMENTS
The authors thank to Dr. Cs. Lila Castellanos Serra for the assistance and advice while preparing the manuscript.
REFERENCES
1. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893-902.
2. González-Fernández A, Faro J, Fernández C. Immune renponses to polysaccharides: Lessons from humans and mice. Vaccine. 2008;26:292-300.
3. Lucas AH, Rittenhouse-Olson K, Kronenberg M, Apicella MA, Wang D, Schreiber JR, et al. Carbohydrate moieties as vaccine candidates: Meeting summary. Vaccine. 2010;28(4):1121-31.
4. Overturf GD. American Academy of Pediatrics. Committee on Infectious Diseases. Technical report: prevention of pneumococcal infections, including the use of pneumococcal conjugate and polysaccharide vaccines and antibiotic prophylaxis. Pediatrics. 2000;106(2 Pt 1):367-76.
5. Organización Panamericana de la Salud. Informe Regional de SIREVA II: datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores, 2000-2005. Documentos Técnicos. Tecnologías Esenciales de Salud. THS/EV-2007/002.
6. Rodgers GL, Arguedas A, Cohen R, Dagan R. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: Potential implications for pneumococcal conjugate vaccines. Vaccine. 2009;27:3802-10.
7. Oosterhuis-Kafeja F, Beutels P, Van Damme P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998-2006). Vaccine. 2007;25(12):2194-212.
8. Rückinger S, Dagan R, Albers L, Schönberger K, von Kries R. Immunogenicity of pneumococcal conjugate vaccines in infants after two or three primary vaccinations: a systematic review and meta-analysis. Vaccine. 2011;29(52):9600-6.
9. Kenne L, Lindberg B, Madden J. Structural studies of the capsular antigen from Streptococcus pneumonia Type 26. Carbohydr Res. 1979;73:175-82.
10. Sun Y, Park MK, Diamond B, Solomon A, Nahm MH. Repertoire of human antibodies against the polysaccharide capsule of Streptococcus pneumoniae serotype 6B. Infect Immun. 1999;67:1172-9.
11. Peeters CC, Lagerman PR, de Weers O, Oomen LA, Hoogerhout P, Beurret M, et al. Preparation of polysaccharide-conjugate vaccines. Methods Mol Med. 2003;87:153-74.
12. Brückner J. Estimation of monosaccharides by the orcinol-sulphuric acid reaction. Biochem J. 1955;60(2):200-5.
13. Porro M, Viti S, Antoni G, Neri P. Modifications of the Park-Johnson Ferricyanide submicromethod for the assay of reducing groups in carbohydrates. Anal Biochem. 1981;118:301-6.
14. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-75.
15. van Dam JE, Breg J, Komen R, Kamerling JP, Vliegenthart JF. Isolation and structural studies of phosphate-containing oligosaccharides from alkaline and acid hydrolysates of Streptococcus pneumoniae type 6B capsular polysaccharide. Carbohydr Res. 1989;187(2):267-86.
16. Chang J, Serrano Y, Garrido R, Rodríguez LM, Pedroso J, Cardoso F, et al. Relevance of O-acetyl and phosphoglycerol groups for the antigenicity of Streptococcus pneumoniae serotype 18C capsular polysaccharide. Vaccine. 2012;30(49):7090-6.
17. Anttila M, Eskola J, Ahman H, Käyhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998;177(6):1614-21.
18. Abeygunawardana C, Williams TC, Summer JS, Hennessey JP. Development and validation of an NMR-based identity assay for bacterial polysaccharides. Anal Biochem. 2000;279:226-40.
19. Kamerling J. Pneumococcal Polysaccharides: A Chemical View. In: Streptococcus pneumoniae. Molecular biology & mechanisms of disease. New York: Mary Ann Liebert, Inc.; 1999. p. 81-112.
20. Hausdorff WP, Siber JR, Paradiso PR, inventors; Wyeth Lederley, Inc., assignee. Multivalent pneumococcal polysaccharide-protein conjugate composition. US patent US 0130137. 2009 May 21.
21. Bröker M, Dull PM, Rappuoli R, Costantino P. Chemistry of a new investigational quadrivalent meningococcal conjugate vaccine that is immunogenic at all ages. Vaccine. 2009;27:5574-80.
22. Rodríguez ME, van den Dobbelsteen GP, Oomen LA, de Weers O, van Buren L, Beurret M, et al. Immunogenicity of Streptococcus pneumoniae type 6B and 14 polysaccharide-tetanus toxoid conjugates and the effect of uncoupled polysaccharide on the antigen-specific immune response. Vaccine. 1998;16(20):1941-9.
23. Wessels MR, Muñoz A, Kasper DL. A model of high-affinity antibody binding to type III group B Streptococcus capsular polysaccharide. Proc Natl Acad Sci USA. 1987;84(24):9170-4.
24. Zou W, Mackenzie R, Thérien L, Hirama T, Yang Q, Gidney MA, et al. Conformational epitope of the type III group B Streptococcus capsular polysaccharide. J Immunol. 1999;163(2):820-5.
25. Moore SL, Uitz C, Ling CC, Bundle DR, Fusco PC, Michon F. Epitope specificities of the group Y and W-135 polysaccharides of Neisseria meningitidis. Clin Vaccine Immunol. 2007;14(10):1311-7.
26. Wessels MR, Kasper DL. Antibody Recognition of the type 14 pneumococcal capsule. evidence of conformational epitope in a neutral polysaccharide. J Exp Med. 1989;169(6):2121-31.
27. Mawas F, Niggemann J, Jones C, Corbel MJ, Kamerling JP, Vliegenthart JF. Immunogenicity in a mouse model of a conjugate vaccine made with a synthetic single repeating unit of type 14 pneumococcal polysaccharide coupled to CRM197. Infect Immun. 2002;70(9):5107-14.
28. Laferriere CA, Sood RK, de Muys JM, Michon F, Jennings HJ. Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect Immun. 1998;66(6):2441-6.
29. Kim JS, Laskowich ER, Michon F, Kaiser RE, Arumugham RG. Monitoring activation sites on polysaccharides by GC-MS. Anal Biochem. 2006;358(1):136-42.
30. Gudlavalleti SK, Lee CH, Norris SE, Paul-Satyaseela S, Vann WF, Frasch CE. Comparison of Neisseria meningitidis serogroup W135 polysaccharide-tetanus toxoid conjugate vaccines made by periodate activation of O-acetylated, non-O-acetylated and chemically de-O-acetylated polysaccharide. Vaccine. 2007;25(46):7972-80.
31. Wessels MR, Paoletti LC, Guttormsen HK, Michon F, D'Ambra AJ, Kasper DL. Structural properties of group b streptococcal type III polysaccharide conjugate vaccines that influence immunogenicity and efficacy. Infect Immun. 1998;66(5):2186-92.
32. Michon F, Uitz C, Sarar A, D'Ambra AJ, Laude-Sharp M, Moore S, Fusco PC. Group B Streptococcal Type II and III Conjugate Vaccines. Physicochemical Properties that Influence Immunogenicity. Clin Vaccine Immunol. 2006;13(18):936-43.
33. Wuorimaa T, Dagan R, Väkeväinen M, Bailleux F, Haikala R, Yaich M, et al. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J Infect Dis. 2001;184(9):1211-5.
34. Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67:2366-70.
35. Daum RS, Hogerman D, Rennels MB, Bewley K, Malinoski F, Rothstein E, et al. Infant immunization with pneumococcal CRM197 vaccines: effect of saccharide size on immunogenicity and interactions with simultaneously administered vaccines. J Infect Dis. 1997;176(2):445-55.
36. An SJ, Yeon YK, Kothari S, Kothari N, Kim JA, Lee E, et al. Physico-chemical properties of Salmonella typhi Vi polysaccharide-diftheria toxoid conjugate vaccines affect immunogenicity. Vaccine. 2011;29(44):7618-23.
37. Dick WE Jr, Beurret M. Glycoconjugates of bacterial carbohydrate antigens. A survey and considerations of design and preparation factors. Contrib Microbiol Immunol. 1989;10:48-114.
38. Laferrière CA, Sood RK, de Muys JM, Michon F, Jennings HJ. The synthesis of Streptococcus pneumoniae polysaccharide-tetanus toxoid conjugates and the effect of chain length on immunogenicity. Vaccine. 1997;5(2):179-86.
39. Mäkelä O, Péterfy F, Outschoorn IG, Richter AW, Seppälä I. Immunogenic properties of alpha (1--6) dextran, its protein conjugates, and conjugates of its breakdown products in mice. Scand J Immunol. 1984;19(6):541-50.
Received in January, 2013.
Accepted in April, 2013.
Jean P Soubal. Departamento de Glicoconjugación. Centro de Química Biomolecular, CQB. Ave. 21 y Calle 200, Reparto Atabey, Apdo. 16042, Municipio Playa, CP 11600, La Habana, Cuba. E-mail: jean.pierre@cqb.cu.