INTRODUCTION
Black gram (Vigna mungo L. Hepper), also known as urdbean, believed to have originated in India is under cultivation from ancient times (Purseglove, 1974). It is an important rainfed short duration legume crop of India and considered as a major source of dietary protein (Abraham et al., 2013). It is also grown in Pakistan, Srilanka, Bangladesh, countries of South East Asia, Africa and America. In India, during 2015-16, urdbean was grown over an area of 3.62 million hectares with the production and productivity of 1.94 million tones and 537 kg ha-1, respectively. It is grown in various agro-ecological conditions and cropping systems with diverse agricultural practices both in rainy (Kharif) and post-rainy (Rabi) seasons (Gupta et al., 2001; Singh and Ahlawat, 2005). Rainy season records higher yields than post rainy due to conducive environment viz., high day temperature, less moisture stress, adequate solar radiation etc. for crop growth and yield. In coastal ecosystems, it is grown in rice fallows with residual moisture after the harvest of paddy.
Urdbean is generally eaten as an accompanying dish with cereal preparations like rice (Oryza sativa L.) and chapatti (Triticum aestivum L.). It is also consumed in the form of ‘dal’ (whole or split, husked/un-husked) or perched. Urdbean is very rich in protein containing about 24% in its seed. Analysis of amino acid profile shows that seed protein of urdbean has a low concentration of sulphur containing amino acid such as methionine and cysteine (Srivastava and Ali, 2004). Comparatively higher lysine content makes it an excellent complement to cereals in terms of balanced human nutrition (Tsou et al., 1979). Green pods are directly consumed as a vegetable by the bulk populace. It is also prescribed as medicine both externally and internally in paralysis, rheumatism and certain diseases of the human nervous system (Singh, 1982) besides being recommended as medicinal diet to cure flatulence due to its easier digestibility. It also provides nutritious green fodder and feeds to the livestock.
The increasing demands for food supply due to the exponential rate of population growth necessitate the acceleration of agricultural output globally. Grain legumes are an essential part of dietary requirements, especially in South Asian and African countries, due to their tremendous nutritional value and wide environmental adaptability. Therefore, induction of genetic variation in high potential grain legumes like black gram is necessary in the present unstable agro-climatic condition for sustained availability of improved varieties with high yield and wide adaptability to farmers (Goyal et al., 2019).
Mutation breeding using individual and combined treatments of various physical and chemical mutagens has been employed successfully in genetic improvement of several grain legumes (Laskar, 2018; Laskar et al., 2019).
Induced mutations may be resorted to developing superior genotypes due to their direct or cumulative effect on genetic background (Baisakh et al., 2011). Mutagenic concentration is directly proportional to both rates of mutation and biological damages (Khursheed et al., 2015). Therefore, it is imperative to optimize the mutagen doses to be used in the mutation breeding experiment for obtaining the maximum rate of mutation with reasonable germination and lesser growth inhibitions. Kozgar et al. (2014) comprehensively documented the important cytogenetic tests for genotoxicity, genetic variation, cytotoxicity and mutagenic potency in induced mutagenesis experiments. Mutagen act directly on the genetic material of the species which in turn alters specific gene expressions and meiosis is directly linked with inheritance of traits through gametes in the subsequent generations (Khursheed et al., 2015). Therefore, the assessment on induced meiotic abnormalities has been considered to be the most reliable indices for accurate estimation of mutagenic potency and genetic sensitivity in applied field of induced mutagenesis (Khan et al., 2015). In higher plants, chromosomal aberrations induced by mutagens have been extensively reported by different authors (Datta and Biswas, 1985; Kaul and Murthy, 1985; Reddy, 1990; Zeerak, 1991; Ahmad, 1993; Saeed, 1993; Sinha and Gandhi, 1994; Kumar and Dubey, 1998; Kumar and Singh, 2003; Kumar and Gupta, 2007; Khan et al., 2015).
Although, cytological studies on major grain legumes are considerable, however detailed reports on urdbean meiotic abnormalities is still limited for access and thus expected to generate new insights for urdbean improvement using induced mutagenesis. With consideration of available facts and findings on urdbean mutagenesis, the present investigation was carried out to estimate the extent of chromosomal aberrations induced at various stages of meiotic division in M1 generation using Gamma rays and EMS alone as well as in combination.
MATERIALS AND METHODS
Plant material
Two varieties of black gram (V. mungo) namely T-9 and Pant U-30 were used in the present study. Seeds of both varieties were obtained from G. B. Pant University of Agriculture and Technology, Pantnagar, Uttaranchal, India. Both varieties are well adapted to agro-climatic conditions of Uttar Pradesh including the site of study viz., Aligarh.
Mutagenic treatments, field seed culture and cytological analysis
Dry seeds of each variety with a moisture content of 12% were irradiated with 100, 200, 300 and 400 Gy doses of Gamma rays from 60Co source at the National Botanical Research Institute, Lucknow, Uttar Pradesh, India. For chemical treatments, healthy seeds of uniform size of each variety were presoaked for 9 hours in distilled water and treated with 0.1, 0.2, 0.3 and 0.4% of EMS (ethylmethane sulphonate-a monofunctional alkylating agent, manufactured by Sissco Research Laboratories Pvt. Ltd., Mumbai, India) for 6 hours with intermittent shaking at room temperature (25±1 ºC). The solution of EMS was prepared in phosphate buffer pH 7. After treatment, the seeds were thoroughly washed in running tap water to remove the excess mutagen from the seed surface.
To understand the relative effect of Gamma rays and EMS, combination treatments were also employed following the procedure adopted by Khursheed et al. (2015). For combination treatments, dry seeds of each variety were first irradiated with Gamma rays at 200 and 300 Gy doses and then treated with 0.2 and 0.3% EMS (i.e. 200 Gy+0.2% EMS, 300 Gy+0.2% EMS, 200 Gy+0.3% EMS and 300 Gy+0.3% EMS). The procedure adopted was similar to that for individual treatment.
Three replications of 100-seeds each were sown for every treatment and control in each variety in a randomized complete block design (RCBD) at the Agriculture Farm, Aligarh Muslim University, Aligarh, India. The spacing was maintained at 30 cm (seed to seed in a row) and 60 cm (between the rows) in the field. The experiment was conducted during summer (Zaid) season of 2008. Recommended agronomic practices were employed for the preparation of field, sowing and subsequent management of the population.
The meiotic analysis was conducted on 30 randomly selected plants from each treatment and control. For meiotic studies, young flower buds were fixed in Carnoy’s fluid (1 part glacial acetic acid: 3 parts chloroform: 6 parts ethyl alcohol) for 24 hours. Ferric chloride was added to the fixative to get the better staining. After 24 hours of fixation, flower buds were transferred to 70% alcohol. Anthers were smeared in 1% acetocarmine solution and pollen mother cells were examined for their behavior at various stages of microsporogenesis under the microscope. The observations were made in a binocular microscope with a magnification of 10X × 100X with oil immersion lens and photographs were taken using MOTIC BA210 fitted with an inbuilt Nikon Camera. The frequencies of meiotic abnormalities were estimated by using the following formula: Frequency of meiotic abnormalities(%)=(Number of abnormal Pollen Mother Cells)/(Total Pollen Mother Cells observed) x 100
RESULTS AND DISCUSSION
In the present study, a broad spectrum of meiotic abnormalities was induced by Gamma rays, EMS and their combination treatments in M1 generation of urdbean (Table 1, Table 2). The spectrum of meiotic abnormalities observed in various mutagenic treatments included univalents, multivalents, laggards, chromatin bridges, micronuclei and chromosome stickiness. In addition, precocious separation of chromosomes at metaphase, unequal separation of bivalents at anaphase, disturbed polarity and cytomixis at telophase were observed at low frequency (Figure 1, Figure 2). Meiosis is genetically the most significant activity of an organism consisting of highly coordinated physiological, biochemical, cytogenetic and phenotypic events which lead to gene recombination, chromosome reduction and gamete formation. Although these changes are continuous, mutation of any of the genes disrupts meiosis and culminates in gametes sterility and other abnormalities. Hence, the study of the meiotic behavior of mutagenized population forms a reliable index for estimating the genotype specific potency of mutagens (Khan et al., 2015).
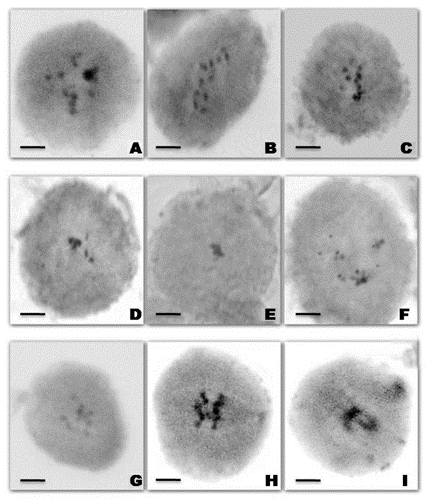
Figure 1. Pollen Mother Cells showing meiotic abnormalities in the treated population of urdbean. (A) Diakinesis with 11 bivalents; (B) Metaphase-I with 11 bivalents; (C) Metaphase-I with univalents; (D) Metaphase-I showing stickiness of chromosomes; (E) Metaphase-I showing stickiness of chromosomes; (F) Metaphase-I with 3I+6II+1III+1IV; (G) Metaphase-I showing precocious movement of chromosomes; (H)Anaphase-I showing movement of chromosomes towards the poles; (I) Anaphase-I showing chromosomes stickiness with difficulty to segregate. Bars = 10 μm.
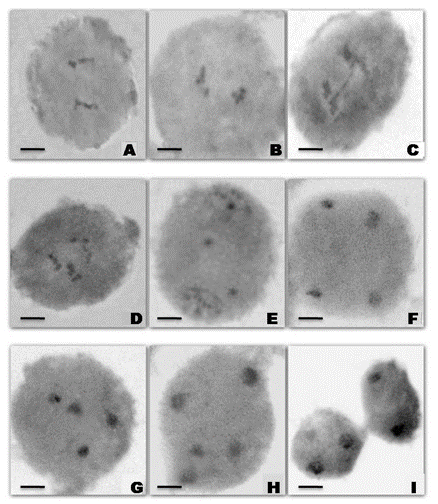
Figure 2. Pollen Mother Cells showing meiotic abnormalities in the treated population of urdbean. (A) Anaphase-I (Control); (B) Anaphase-I with lagging chromosome; (C) Anaphase-I showing chromatin bridge; (D) Anaphase-I showing unequal separation of chromosomes; (E) Telophase-I with lagging chromosomes; (F) Telophase-II (Control); (G) Telophase-II showing disturbed polarity; (H) Telophase-II showing eliminated chromosomes forming micronucleus; (I) Telophase-I showing cytomixis. Bars = 10 μm
Almost all the mutagenic treatments induced a high degree of stickiness among the bivalents which adversely affected the normal disjunction. Stickiness has been reported to be the result of partial dissociation of nucleoproteins and alteration in the pattern of organization of chromosomes by Evans (1962), while Rao and Laxmi (1980) attributed it to be due to the disturbances of cytochemically balanced reactions by the mutagens. In addition, Gaulden (1987) postulated that stickiness may result from defective functioning of one or two types of specific non-histone proteins involved in chromosome organization which are necessary for chromatid separation and segregation. The altered functioning of these proteins leading to stickiness is caused by mutations in the structural genes coding for them (hereditary stickiness) or by the action of mutagens (induced stickiness).
Chromosome stickiness has been reported to decrease the pollen fertility in some crop species (Rao et al., 1990; Pagliarini et al., 2000). The occurrence of univalents indicates non-homology between certain chromosomes in the compliment. The mutagenic treatments induce structural changes in some of the chromosomes which restrict the pairing and hence the formation of univalents. Reduction in chromosome pairing has also been attributed to mutations in the genes governing homologous chromosome pairing and/or chromosomal structural changes (Gottschalk and Villalobes-Pietrini, 1965; Reddy et al., 1991; Singh, 1992). At metaphase-I, some of the univalents disjuncted early. Such chromosomal divergences in the form of precocious movements are pointed towards the structural differentiation of the homologous pair. Stickiness of chromosomes at metaphase-I adversely affected the normal disjunctions of chromosomes at anaphase-I, which resulted in the formation of laggards and unequal separation of chromosomes at anaphase stage. The laggards observed, in the present study, have also been reported earlier and may be the result of delayed terminalization, stickiness of chromosome or the failure of chromosomal movement (Jayabalan and Rao, 1987).
Such abnormalities lead to micronuclei formation (Zeerak and Zargar, 1998) and non functional spores. The frequency of micronuclei was less at telophase-II as compared to the frequency of univalents and laggards, indicating that some chromosomes were included in the main nucleus. This seems to be the normal behavior of many species (Koduru and Rao, 1981). Unequal separation of chromosomes would lead to the production of aneuploid gametes (Zeerak, 1992). Chromosome bridges were frequently observed at anaphase-I. Chromosome breakage and reunion of broken ends could lead to the formation of bridges (Ignacimuthu and Babu, 1989). Anaphase bridges were also recorded by Suganthi and Reddy (1992) in Triticum aestivum, Zeerak (1992) in Lycopersicon esculentum L., Fatma and Khan (2009) in Vicia faba and Khan and Tyagi (2009) in Glycine max L.
Multivalent formation in mutagenic treated plants has been attributed to many breaks induced by mutagens which may lead to reciprocal translocation and resulting in multivalent formation. Such type of multivalent formation has also been reported in brinjal (Solanum melongena L.) (Satyanarayana and Subhash, 1982), urd and mung beans (Vigna radiata L. Wilczek) (Ignacimuthu and Babu, 1989), rice (Oryza sativa L.) (Radhadevi and Reddi, 1997) and chilli (Capsicum frutescens) (Dhamayanthi and Reddy, 2000). In the present study, among individual mutagenic treatments, Gamma rays produced a higher number of multivalents. Gamma rays and EMS treatments have been reported to induce the formation of multivalents in V. radiata (Ignacimuthu and Sakthivel, 1989) and Lens culinaris Medik (Reddy and Annadurai, 1992).
Disturbed polarity at telophase-II could be due to alteration in the gene controlling biochemical pathways of the substance that determine the position of spindle poles. Similar reports were made in wheat (Triticum aestivum L.) (Suarez and Bullrich, 1990), barley (Hordeum vulgare) (Kumar and Singh, 2003) and faba bean (Vicia faba L.) (Ansari and Ali, 2009). Cytomixis generally refers to the migration of chromatin from one cell to the other through cytoplasmic connections. Although cytoplasmic connections are very common in angiosperms, the movement of nuclear material through them is rare. In the present study, although no visual transfer of chromosome through cytoplasmic connections could be detected, the formation of cytoplasmic channels between the two cells suggests that screening of mutagen treated population for varied ploidy level and its utilization in plant breeding can yield interesting results. Cytomixis may have serious genetic consequences by causing deviations in chromosome number and may represent an additional mechanism for the origin of aneuploidy and polyploidy (Sarvella, 1958; Siddiqui et al., 1979). Cytomixis has been detected at higher frequency in genetically imbalanced species such as hybrids as well as apomictic and polyploid species (Gottschalk, 1970; Yen et al., 1993).
The abnormalities induced were found to be dose-dependent where a linear correlation established between increasing strength of mutagen treatments and increasing frequency of meiotic aberrations (Figure 3). Although the types of chromosomal abnormalities were more or less common in both the varieties, yet the frequency of such aberrations was comparatively more in var. Pant U-30 than the var. T-9 (Table 3, Figure 4) indicating that it is more sensitivity towards the mutagens. Combined treatments were most effective for induction of meiotic aberrations, whereas Gamma rays individually induced more aberrations than EMS (Table 3, Figure 4). These results support the general hypothesis that physical mutagens produce more cytological abnormalities than chemical ones (Kozgar, 2014). However, EMS was earlier found to be more effective in inducing meiotic irregularities than Gamma rays individually as well as in combination with Gamma-ray treatment (Dhamayanthi and Reddy, 2000). Dose dependent increase in meiotic abnormalities has also been reported by Ignacimuthu and Babu (1989) in urdbean (V. mungo) and mung beans (V. radiata) and Dhamayanthi and Reddy (2000) in chilli (Capsicum annuum L.).

Figure 3. Dose dependent linear relationship between chromosomal aberrations frequency and mutagen treatment concentrations in urdbean (Vigna mungo L. Hepper).
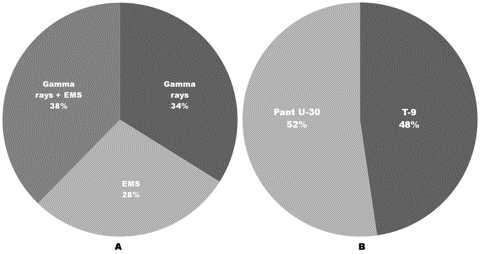
Figure 4. Relative total frequency (%) of meiotic abnormalities induced in urdbean (Vigna mungo L. Hepper). A: Comparison between Gamma rays, EMS and their combination; B: Comparison between urdbean varieties Pant U-30 and T-9.
The scarcity of available genetic variations is among the prime constraints in black gram breeding, thus induced mutagenesis is one of the most preferred complimentary technique for generation of accessible mutation(s) in the black gram genotype(s). In mutation breeding, the selection of suitable mutagens agents and their doses are prerequisite for subsequent generation of mutagenised population(s) and genotypic improvement of any crop. In the present study, meiotic analysis of induced chromosomal aberrations facilitated the process of mutagen potency assessment in two widely cultivated black gram genotypes. The wide range of induced chromosomal aberrations at different treatment doses and the aberrations frequencies estimated in the study showed higher mutation rates with lower lethality can be obtained by employing physical (Gamma rays) and chemical (EMS) mutagens agents together at lower doses. The meiotic aberrations resulted directly from the interactions between applied mutagen doses and black gram chromosomes. Therefore, it provides authentic information about the genotypic tolerable limits at which useful mutations can be induced by employing suitable Gamma rays and EMS treatments in black gram. Moreover, it implies that the mutagenic populations generated in both T-9 and Pant U-30 varieties using Gamma rays and EMS alone as well as in combination are useful source for isolation of early putative mutants through mutation breeding in subsequent generations.
Table 1. Frequency and spectrum of meiotic abnormalities in various mutagenic treatments in M1 generation of black gram (Vigna mungo L. Hepper) var. T-9.
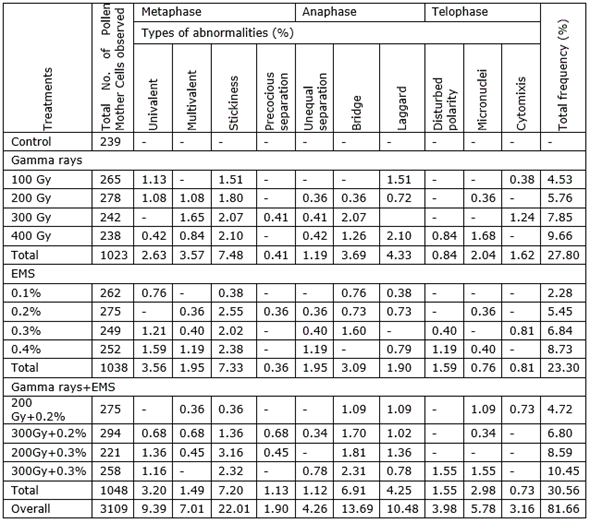
Table 2. Frequency and spectrum of meiotic abnormalities in various mutagenic treatments in M1 generation of black gram (Vigna mungo L. Hepper) var. Pant U-30.
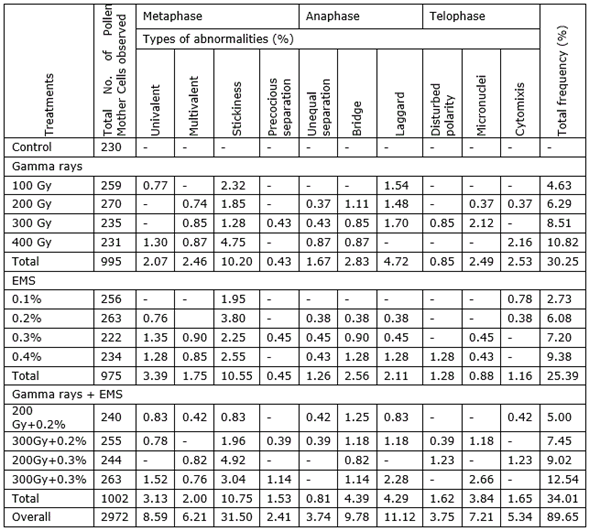
Table 3. Total (pooled) frequency of meiotic abnormalities induced by Gamma rays, EMS and their combinations in M1 generation of black gram (Vigna mungo L. Hepper).
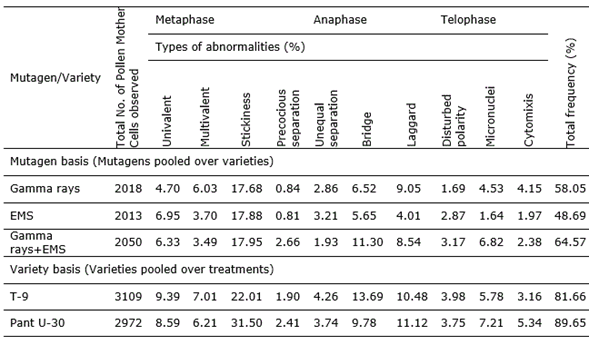
In the present study, broad array of chromosomal aberrations have been induced at various stages of meiotic division in M1 generation using Gamma rays and EMS alone as well as in combination. The combined mutagen agent doses resulted in higher frequency of abnormalities as compared to the individual doses of Gamma rays and EMS in both the varieties Pant U-40 and T-9. However Pant U-40 reflected higher mutagenic sensitivity than the var. T-9. Differential sensitivity of the varieties, observed in the present study, suggested that mutagenic potency completely depends on the genetic makeup of the target species and cannot be generalize for all biological systems. Hence, prior selection of varieties, mutagen agent doses and durations are the key to achieve a desirable spectrum of mutation. Comparative estimation of induced cytological abnormalities suggested higher mutagenic sensitivity of var. Pant U-30 than the var. T-9 towards the treatments used. This reflects a relatively broader genetic tolerance level of black gram var. T-9.
CONCLUSIONS
Combined treatments of Gamma rays and EMS are most effective for induction of meiotic aberrations in black gram (Vigna mungo L. Hepper) varieties Pant U-30 and T-9, whereas individually Gamma rays induce more aberrations. The higher frequency and wide spectrum of aberrations induced in combination treatments suggests physical and chemical mutagens when employed together at predetermined tolerable doses could increase the rate of mutations in black gram genotypes. Also, the meiotic anomalies observed in the study suggests associated alteration of gene(s) expression in the mutagenised populations that may govern the trait(s) of the interest of breeder which could get stabilized in advanced generations.














