Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Ciencias Médicas de Pinar del Río
versión On-line ISSN 1561-3194
Rev Ciencias Médicas vol.27 no.3 Pinar del Río mayo.-jun. 2023 Epub 01-Mayo-2023
Articles
Lofgren's síndrome as a rare form of acute sarcoidosis
1University of Medical Sciences of Pinar del Río. "Augusto César Sandino" Municipal Hospital. Pinar del Río, Cuba.
2National Institute of Endocrinology. Havana, Cuba.
3University of Medical Sciences of Pinar del Río."Dr. León Cuervo Rubio" Clinical Surgical Teaching Hospital. Pinar del Río, Cuba.
Introduction:
sarcoidosis is a granulomatous and multisystemic disease of unknown etiology defined as an inflammatory response to an environmental antigen in a genetically susceptible person. Löfgren's syndrome is a form of presentation with stereotypical clinical findings manifested as a triad of acute periarthritis, erythema nodosum and hilar adenopathy.
Case report:
we report a case with the characteristic findings of this clinical form in which, after the multidisciplinary discussion of the findings found in the physical examination and complementary studies, it was decided to start treatment with glucocorticoids without performing mediastinal lymph node biopsy, an invasive and risky procedure.
Conclusions:
the clinical evolution of the patient was favorable and at present he is asymptomatic, without treatment and without evidence of complications or progression towards the chronic form of the disease after three years of evolution.
Key words: SARCOIDOSIS; GRANULOMATOUS DISEASE; ERYTHEMA NODOSUM; AUTOIMMUNE DISEASES.
INTRODUCTION
The initial description of sarcoidosis was made by Jonathan Hutchinson, who in 1877 reported a patient with multiple brownish, patchy, raised skin lesions. Subsequently, Caesar Boeck described "multiple benign sarcoid lesions of the skin", using the term "sarcoid" because, although these lesions resembled a sarcoma, they were benign.1
Löfgren's syndrome is an acute presentation of sarcoidosis characterized by bilateral hilar adenomegaly, fever and polyarthritis. Sarcoidosis is a multisystem disease of unknown etiology that can affect any organ characterized by the presence of non-caseating granulomas in biopsies. Several forms of presentation are described, most of them probably asymptomatic. Latin America is a region with a low incidence of sarcoidosis, probably due to genetic differences and environmental exposure to certain antigens or due to underreporting of this condition and the high prevalence of other endemic granulomatous diseases (tuberculosis, leprosy, deep mycosis) that mask the diagnosis.1
In Pinar del Río, a rare case of solitary sarcoidosis of the spleen was reported in a 58-year-old female patient who presented a clinical picture suspicious of malignant splenic neoplasm; diagnostic and therapeutic splenectomy was performed, with the presumptive diagnosis of primitive lymphoma of the spleen and the pathological anatomy revealed multiple non-caseifying splenic granulomas.2
The aim of the present work is to describe a case of sarcoidosis in its acute and reversible form of Löfgren's syndrome. The relevance of this report is given by its rare form and the clear clinical definition of its presentation that allowed assuming its diagnosis even in the absence of histological study to confirm it, which was demonstrated by the therapeutic response and its favorable evolution.
CASE PRESENTATION
Patient 48 years old, with white skin and health history, started with asymmetric volume increase in malleolar region of both lower limbs. Ten days later she developed erythematous nodular hardening in the lower third of the right leg and another in the left ankle, initially painless and later with pain on deep palpation. He also presented non-productive cough, slight respiratory distress and other general manifestations such as decay, easy tiredness and afternoon fever.
On physical examination the body weight was 79 kg, with a height of 1.70 cm. Mucous membranes were normal-colored and moist; two nodules of approximately 2 cm were palpated on the skin, located in the distal and antero medial third of the right lower limb and on the left ankle, with erythema and pain on palpation. She had non-painful asymmetric malleolar edema, with no skin color changes.
Laboratory studies at the first consultation showed hemogram with slight leukocytosis; blood chemistry, C-reactive protein, rheumatoid factor, HBag, AcC and HIV were negative and serology was non-reactive. During three admissions in the following three months, evolutionary studies were repeated and others were indicated; the peripheral lamina examination with normochromia and neutrophilia and triglycerides with a range between 2,80-5.61 mmol/L were positive. The rest of the investigations were normal (Table 1).
Echocardiogram and fundus examination were normal.
Ultrasound of the nodular areas showed oval, echogenic images of regular capsule and homogeneous texture measuring approximately 17 x 8 mm and 6 x 3 mm respectively, located 2 mm below the skin.
Abdominal ultrasound showed absence of visceromegaly, with slightly increased hepatic echogenicity, absence of adenopathy and no intra-abdominal free fluid, and a 5 mm lithiasis was found in the middle caliceal group without ectasia. The ultrasound of the neck showed small lymphadenopathies of inflammatory aspect in the posterior cervical chain and in projection of the base of the neck to the left, close to the aortic arch, an 8 mm lymphadenopathy of inflammatory aspect was visualized, with a 3 mm cortex.
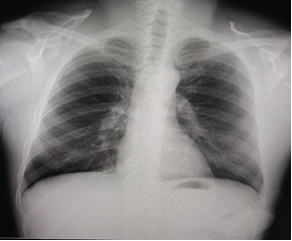
Chest X-ray showed a normal cardio-thoracic index, showing bilateral hilar thickening with widening of the superior mediastinum with polycyclic contours due to the presence of lymphadenopathy (Fig. 1), which suggested the diagnosis of sarcoidosis. Lung fields showed no alterations. A simple and contrasted chest CT scan was indicated, whose image supported the initial impression.
The simple chest CT showed mediastinal widening with multiple peritrachial lymphadenopathies, which in the contrast study were not enhanced, being the largest (41 mm) close to the left subclavian artery, which due to its characteristics could be related to sarcoidosis or lymphoproliferative disease.
Positron emission tomography revealed multiple mediastinal adenopathies and both pulmonary hilar with glycolytic hypermetabolism, and a lesion at the level of the greater cisura compatible with segmental adenopathy. Late imaging showed a discrete increase in the glycolytic activity of some of these lesions. The interpretation of this result defined that "the areas of glycolytic hypermetabolism described seem to be related to a benign inflammatory process, and other etiology, mainly lymphoproliferative disease, cannot be ruled out".
Table 1 Results of laboratory studies
| Complementary Results | Resultados | |
|---|---|---|
| First consultation | Admission range | |
| Hemoglobin | 15,4-13,3 g/L | |
| Hematocrit | 0,45L/L | 0,41-0,44 L/L |
| Leukocyte count | 12x109/L | 6,4-11,6 x 109/L |
| Platelets | 40mm/h | 229-242 x 109/L |
| Erythrocyte sedimentation rate | 22-26 mm/h | |
| Peripheral lamina | normochromia, neutrophilia, platelets adequate in number with clumping | |
| Creatinine | 95,6 umol/L | 102-118 µmol/L |
| Cholesterol | 3,87 mmol/L | 4,35-8,40 mmol/L |
| Triglycerides | 4,45 mmol/L | 2,80-5,61 mmol/L |
| TGP 1 | 30,2 U/I | 28,3-41,2 U/l |
| TGO 2 | 20,1 U/I | 14-24,9 U/l |
| GGT 3 | 16,5-21 U/I | |
| CPK 4 | 47 U/I | |
| LDH 5 | 372U/I | |
| Alkaline phosphatase | 56,3-284,2 U/L | |
| Total protein | 61,3-74 g/L | |
| Uric acid | 393umol/L | 391,6-415 µmol/L |
| Glycemia | 3,54-5,61 mmol/L | |
| PTG 6 | Fasting: 5,40 mmol/L 2hours: 9,28 mmol/L | |
| Amylase | 76,2 U/L | 59,8-71,8 U/I |
| Urea | 3,73 umol/L | 4,40 mmol/L |
| Serum calcium | 1,94 mmol/L | 2,3 mmol/L |
| Phosphorus | 1,48 mmol/L | 1,05 mmol/L |
| Negative C-reactive protein | Negative | 9,23 mg/L |
| Rheumatoid factor | 9,38 mg/L | |
| PSA 7 | 1,04 ng/L | |
| Feces | Negativo | |
| Urine partial Normal | Normal | |
| ANCA 8 | Negative | |
| ANA 9 | Negative | |
| HBAg 10 | Negative | Negative |
| AcC 4 | Negative | Negative |
Legend: 1/TGP: glutamic-pyruvic transaminase; 2/TGO: glutamic-oxaloacetic transaminase; 3/GGT: gamma glutamyl transpeptidase; 4/CPK: creatine phosphokinase; 5/LDH: lactate dehydrogenase; 6/PTG: glucose tolerance test; 7/PSA: prostate surface antigen; 8/ANCA: prostate surface antigen; 9/ANCA: prostate surface antigen; 10/ANCA: prostate surface antigen; 11/ANCA: prostate surface antigen; 12/ANCA: prostate surface antigen; 13/ANCA: prostatic surface antigen: prostatic surface antigen; 8/ANCA: neutrophil anti-cytoplasmic antibody; 9/ANA: antinuclear antibody; 10/HBAg: hepatitis B surface antigen; 11/AcC: hepatitis C antibody; 11/AcC: hepatitis C antibody.
The physical-chemical study of urine showed increased calcium excretion, increased Ca/Cr ratio, weakly acidic pH, with sodium of 131 mmol/l.
The biopsy performed on the subcutaneous nodule of the right leg showed the presence of panniculitis and erythema nodosum, with no signs of malignancy.
In the multidisciplinary discussion it was decided not to perform the lymph node biopsy because the clinical manifestations were highly suggestive of sarcoidosis and the imaging studies strongly supported the diagnosis.
It was decided to start treatment with oral prednisone (60 mg/day) for 21 days and then gradually reduce the dose to re-evaluate in one month and, according to the clinical evolution and therapeutic response, decide whether or not to perform mediastinal lymph node biopsy.
After one month of evolution with treatment, a simple chest CT scan was repeated, which reported the existence of homogeneous lung fields, without defining mediastinal adenomegaly. It was then decided, due to the favorable evolution, not to perform a lymph node biopsy and to maintain treatment with prednisone (40 mg/day) and re-evaluate.
Two months later, the patient showed clinical and radiological improvement and it was decided to reduce the steroid dose, with maintenance of 5 mg/day.
A repeat CT scan of the chest six months later showed the presence of a 1,3 cm lymphadenopathy at the level of the supra-aortic vessels, without defining a focal nodular lesion or parenchymal alterations. In this study no adenopathy was defined at the level of the hilae.
Prednisone treatment was maintained for 22 months until total withdrawal. Two years and nine months after diagnosis, including 10 months asymptomatic without treatment, he was readmitted for laboratory tests, which showed as the only positive result a mild neutropenia, in contrast to the neutrophilia he presented at diagnosis and maintained during steroid treatment.
The evolving chest X-ray ( 1,Fig. 2) showed radiological improvement with discrete bilateral hilar thickening predominantly on the right, slight accentuation of the basal hilum and very fine fibrotic bands in both lung fields. Currently, the patient is in good general condition without treatment and periodic follow-up every six months.
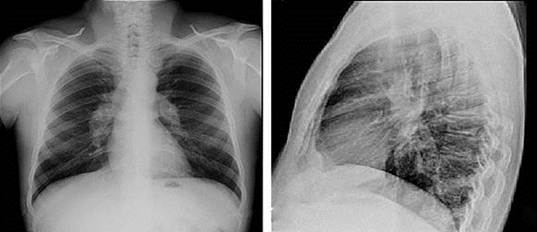
Fig. 1 Chest X-ray (posterior and lateral antero) at the time of diagnosis with bilateral hilar lymphadenopathy, without the presence of pulmonary infiltrates or fibrosis.
DISCUSSION
The clustering of sarcoidosis in families and communities suggests genetic predisposition, shared exposures or interpersonal transmission (less likely).1
Its defining finding, non-caseating granulomas, are the result of a response to an antigen and develop most frequently in the lungs and lymph nodes and can affect any organ.3
Clinical manifestations depend on organ involvement and vary over time from spontaneous remission to chronic disease. Pulmonary involvement (dyspnea, cough, chest discomfort and crackles) is 90 % and extrapulmonary involvement (general malaise, weakness, anorexia, weight loss and fever) is 95 %.1,3
Löfgren's syndrome is an acute form of sarcoidosis. The triad of periarthritis, erythema nodosum and hilar adenopathies has a specificity of 95 %, which allows a clinical diagnosis without biopsy, as it happened in the reported patient. Acute polyarthritis exists in 10 to 40 % of patients, bilateral hilar lymphadenopathy and erythema nodosum are almost invariably present in the early stages, and fever is present in 60 to 90 % of cases. Arthritis is oligoarticular in more than 80 % and often constitutes the first manifestation, being typically symmetrical, affecting mainly the ankle joint.4
Sarcoidosis is often suspected when bilateral hilar adenopathies are accidentally detected in a chest X-ray.5) The generalization of endoscopic techniques that allow echoendoscopy-guided lymph node biopsy with superior results to mediastinoscopy has significantly changed the diagnostic procedure.6) Due to its low invasiveness and low risk, in case of doubts about the "typicality" of the clinical or imaging manifestations, it is always better to perform it, but if the biopsy is not performed, there must be certainty of a systematic follow-up and this decision must be agreed with the patient.3,6
When a biopsy is decided, it should preferably be of other lesions such as cutaneous lesions (modified scars or on a tattoo) as well as peripheral lymphadenopathy and oral lesions.3) Cutaneous lesions exclude erythema nodosum, in which histology only shows panniculitis-like alterations, a finding that corresponds to what happened in the reported case.
The 2020 guidelines for the diagnosis of sarcoidosis,3) recommend not performing lymph node biopsies if there is a high probability of sarcoidosis, a criterion that was followed in the patient.
When sarcoidosis is clinically suspected, but not proven at the site of biopsy, positron emission tomography can help to identify its existence.7) When this procedure was performed, mediastinal adenopathies and a segmental pulmonary one with the appearance of a benign inflammatory process were identified, which reinforced the diagnostic criteria and contributed to the decision to initiate treatment without performing mediastinal lymph node biopsy.
The reported patient is an example of this procedure. He presented manifestations suggestive of the disease and the chest X-ray, as well as other laboratory and imaging studies, supported sufficient criteria to establish the diagnosis, being approved by a multidisciplinary group and with the patient's informed consent not to perform the histological study.
The evaluation of the progress of the disease depends fundamentally on the degree of respiratory compromise that is evaluated by means of respiratory functional tests.1,5) The absence of respiratory symptoms, the negative examination of the lungs and the improvement of the images with treatment have not made it necessary to perform these studies so far.
Laboratory tests play an essential role in diagnosis and the degree of organ involvement in deciding therapeutic options.3,8 The CBC may show anemia, eosinophilia and leukopenia, while elevated azotemia and liver enzymes are useful to identify kidney and liver involvement. Total proteins may be elevated due to hyperganmaglobulinemia and erythrocyte sedimentation rate is commonly nonspecifically slightly accelerated.1,5) The reported case presented only mild neutropenia in one of the blood counts, elevated total proteins and slightly increased erythrocyte sedimentation rate. No findings were found to indicate renal, hepatic, cardiac or neurologic dysfunction, consistent with a diagnosis of one of the acute forms of the disease.
Sarcoidosis is a cause of hypercalcemia that is often not thought of and its elevation in serum and 24-hour urine strongly indicates the diagnosis if associated with a suggestive clinical picture.1) In the patient described, serum calcium was normal, however, it showed an elevation of its urinary excretion with increased Ca/Cr ratio and nephrolithiasis.
Although spontaneous remission of this clinical form is frequent, in many cases the use of corticosteroids is required at some point.8) The prognosis after recovery is excellent. In an investigation conducted two years after the onset of the disease, 8 % of patients had evidence of activity and only 6 % had relapses.9) The reported case exemplifies this statement.
For the treatment of Löfgren's syndrome, it is recommended to start with non-steroidal anti-inflammatory drugs, since acute arthritis is usually self-limiting. After two weeks without response, treatment with corticosteroids is the first line treatment.10
The effects of steroids consist of controlling inflammation and modulating the immune response.11) In sarcoidosis there is an elevation of proinflammatory cytokines that condition alterations of the hypothalamus-pituitary-adrenal axis and a decrease in endogenous glucocorticoid levels. The aim of their use is to reduce granulomatous inflammation, but the recognition of other clinical manifestations is necessary to obtain optimal results in patients with advanced stages.11,12
Currently, several novel treatment avenues are under investigation; of which, corticosteroid-sparing antisarcoidosis drugs are necessary for the management of non-granulomatous complications of pulmonary sarcoidosis, with possible optimal outcomes in patients with advanced disease.13
This patient with Löfgren's syndrome is of interest because it is a rare disease that is usually not recognized due to its similarity to other more common entities and because its recovery can be spontaneous. Likewise, since its clinical manifestations and the results of the studies were highly suggestive of the diagnosis, it was not essential to perform a lymph node biopsy to certify the diagnosis and the satisfactory therapeutic response becomes a criterion for diagnostic reaffirmation.
BIBLIOGRAPHIC REFERENCES
1. Jain R, Yadav D, Puranik N, Guleria R, Jin JO. Sarcoidosis: Causes, Diagnosis, Clinical Features, and Treatments. J Clin Med1. [Internet]. 2020[Citado 20/05/2023]; 9(4):1081. Disponible en: Disponible en: https://doi.org/10.3390/jcm9041081 1. [ Links ]
2. Martínez RW, Gil LG, Forteza TO, Borrajero MI. Sarcoidosis esplénica. Presentación de un caso. Breve revisión de la literatura. Revista Electrónica de Autopsia[Internet].2011[Citado 20/05/2023]; 9: aprox. 11p. Disponible en:Disponible en:https://journaldatabase.info/articles/sarcoidosis_esplenica_presentacion_un.html 2. [ Links ]
3. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med[Internet]. 2020[Citado 20/05/2023]; 201(8):e26-e51. Disponible en: Disponible en: https://doi.org/10.1164/rccm.202002-0251ST 3. [ Links ]
4. Brown F, Modi P, Tanner LS. Lofgren Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing[Internet]; 2021[Citado 20/05/2023]. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/29493940 4. [ Links ]
5. Polverino F, Balestro E, Spagnolo P. Clinical Presentations, Pathogenesis, and Therapy of Sarcoidosis: State of the Art. J Clin Med[Internet]. 2020[Citado 20/05/2023]; 9(8):2363. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/32722050/ 5. [ Links ]
6. Fu Y, Chen Q, Yu Z, et al. Clinical application of ultrasound-guided mediastinal lymph node biopsy through cervical mediastinoscopy. Thorac Cancer[Internet]. 2021[Citado 20/05/2023];12(3):297-303. Disponible en: Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7862788/ 6. [ Links ]
7. Tol SJM, van der Zant FM, Knol RJJ, Wondergem M, Broos WAM. Löfgren Syndrome on 18F-FDG PET/CT: An Acute Manifestation of Sarcoidosis. Clin Nucl Med[Internet]. 2022[Citado 20/05/2023]; 47(1): 61-62. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/34874349/ 7. [ Links ]
8. Gerke AK. Treatment of Sarcoidosis: A Multidisciplinary Approach. Front Immunol[Internet]. 2020[Citado 20/05/2023]; 11: 545413. Disponible en: Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7732561/ 8. [ Links ]
9. Lopez MC, Amadeu TP, Ribeiro-Alves M, et al. Defining prognosis in sarcoidosis. Medicine9. (Baltimore) [Internet]. 2020[Citado 20/05/2023]; 99(48): e23100. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/33235069/ 9. [ Links ]
10. Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J[Internet]. 2021[Citado 20/05/2023]; 58(6): 2004079. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/34140301/ 10. [ Links ]
11. Comité Nacional de Endocrinología. Consideraciones para una corticoterapia segura. Arch. Argent Pediatr[Internet]. 2018[Citado 20/05/2023]; 116(Supl3): S71-S76. Disponible en: Disponible en: https://www.sap.org.ar/docs/publicaciones/archivosarg/2018/v116n3a33s.pdf 11. [ Links ]
12. Campbell A M, Martin J R, Erstad B L. Corticosteroid Tapering Regimens in Rheumatic Disease: A Systematic Review. J Clin Rheumatol[Internet]. 2020[Citado 20/05/2023]; 26(2): 41-47. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/32073513/ 12. [ Links ]
13. Culver DA, Judson MA. New advances in the management of pulmonary sarcoidosis. BMJ13. [Internet]. 2019[Citado 20/05/2023]; 367: l5553. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/31641045/j 13. [ Links ]
Received: March 01, 2022; Accepted: April 09, 2023











 texto en
texto en