Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cultivos Tropicales
versión On-line ISSN 1819-4087
cultrop vol.40 no.1 La Habana ene.-mar. 2019
Original Article
Potyvirus incidence in solanaceus producer areas in west zones of Cuba
1Departamento de Microbiología y Virología, Facultad de Biología, Universidad de La Habana
2Centro Nacional de Sanidad Agropecuaria (CENSA), carretera de Jamaica y Autopista Nacional. Apartado 10, San José de las Lajas, Mayabeque, Cuba
3Grupo Empresarial GEOCUBA. Cuba
Potyvirus genus is one of the most studied among the plant virus group. It is worldwide distributed and affects different hosts and solanaceus crops. The aims of this work was to determinate the Potyvirus incidence in agroeconomical important solanaceus crop in the west zones of Cuba. 1120 young leaves of Solanum lycopersicum, Capsicum annuum and Nicotiana tabacum were collected and analyzed by indirect ELISA, using monoclonal antibodies. Among 34 and 75 % of the plant material was positive. Highest incidence value was detected on San Juan y Martínez and San Antonio de los Baños districts while the lowest values was registered in Güira de Melena
Key words: Solanum lycopersicum; Capsicum annuum; Nicotiana tabacum; Potyvirus; incidence
INTRODUCTION
The potato virus Y (PVY) is one of the most pathogenic species within the genus Potyvirus, family Potyviridae1,2. It has been identified as responsible for large losses in crops fundamental to the economy such as Solanum lycopersicum L., Capsicum annuum L., Solanum tuberosum L. and Nicotiana sp. 3,4. PVY is widely distributed worldwide and is transmitted in a non-persistent and not circulative manner by different species of aphid, which adapt to a wide variety of habitats 1.
It is a naked virus, with a flexible and filamentous morphology, 740 nm long and 11 nm in diameter. It presents a genome consisting of a single strand of RNA of positive polarity, about 9.7 Kb containing two open reading frames flanked by 3'and 5'untranslated regions 5. The symptoms caused by PVY are fundamentally related to a mosaic of color, necrosis and wrinkling of the leaves, although they can vary in correspondence with the viral variant that produces the infection, the characteristics of the host, the time of infection or environmental conditions 6,7.
In the last decade, numerous studies have shown the great genetic and biological diversity of PVY 8, which, together with its wide global distribution, its potential for recombination and its pathogenicity in different environments, represent a challenge for agriculture. Taking into account this background, numerous methodologies have been developed that aim to optimize the early detection of the virus. Within these, techniques based on the polymerase chain reaction are well documented, however, the use of this technique is routinely limited by the number of samples to be evaluated due to the complexity of RNA viral extraction 4,8.
In Cuba, several authors have reported the presence of PVY affecting C. annuum and S. tuberosum in the provinces of Artemisa and Mayabeque (9,10). These studies mainly used traditional methods such as observation of symptoms, with which the existence of circulating PVY strains in different regions of the country is known, but the distribution and incidence of the virus in the different localities producing Solanaceae is unknown. The objective of this work is to determine the incidence of Potyviruses in Solanaceae of agroeconomic importance in the western region of Cuba, using an indirect ELISA.
MATERIALS AND MRTHODS
AREA OBJECT OF STUDY
The coordinates of the 14 localities included in the study, belonging to the provinces of Pinar de Río and Artemisa, were determined, using Global Geo-positioning (GPS) technology with an SR20 equipment (Table 1), according to the procedure described by Ricardo -Desin and collaborators in 2010 11).
Table 1 Geographical location of the area under study
| Locality | Location | Coordenates in WGS-84* | Plane Coordinates (m) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Latitude | Length | X | Y | ||||||
| 1 | Consolación del Sur1 | 23 | 31 | 9,670009 | 83 | 30 | 34,111532 | 241843,44 | 301197,28 |
| 2 | Consolación del Sur1 | 22 | 30 | 17,444713 | 83 | 31 | 30,519203 | 240204,78 | 299618,14 |
| 3 | San Juan y Martínez1 | 22 | 16 | 57,221336 | 83 | 49 | 29,875211 | 208900,19 | 275558,05 |
| 4 | San Juan y Martínez1 | 22 | 16 | 57,046232 | 83 | 49 | 39,903693 | 208613,07 | 275558,05 |
| 5 | San Luis1 | 22 | 16 | 50,142266 | 83 | 46 | 22,478005 | 214259,58 | 275240,72 |
| 6 | Pinar del Río | 22 | 23 | 11,837290 | 83 | 43 | 2,940217 | 220182,28 | 286874,25 |
| 7 | Los Palacios1 | 22 | 35 | 43,434078 | 83 | 15 | 9,317690 | 268393,92 | 309198,97 |
| 8 | Bauta2 | 22 | 59 | 15,338840 | 82 | 32 | 17,761689 | 342279,04 | 351701,52 |
| 9 | San Antonio de los Baños2 | 22 | 51 | 54,533886 | 82 | 28 | 45,747493 | 348184,09 | 338082,51 |
| 10 | San Antonio de los Baños- Lázaro Peña2 | 22 | 52 | 47,276633 | 82 | 28 | 38,070120 | 348418,84 | 339702,63 |
| 11 | Bejucal (Artemisa) | 22 | 52 | 29,473538 | 82 | 22 | 51,527239 | 358291,06 | 339061,35 |
| 12 | San Antonio de los Baños2 | 22 | 50 | 19,409613 | 82 | 29 | 18,672986 | 347216,64 | 335165,93 |
| 13 | San Antonio de los Baños2 | 22 | 51 | 21,038225 | 82 | 28 | 3,096045 | 349389,84 | 337040,36 |
| 14 | Güira de Melena2 | 22 | 47 | 33,225866 | 82 | 32 | 1,805998 | 342513,61 | 330101,18 |
*Coordinates obtained with GPS (grades, minutes, seconds), 1Pinar del Rio, 2Artemisa
TAKING AND PROCESSING OF THE SAMPLES
A total of 1120 samples of young leaves of S. lycopersicum, C. annuum and N. tabacum (symptomatic and asymptomatic) were collected (Table 2) from the taking of plants from 20 specific points of a quadrant of 4x4 m, from according to the design of a closed envelope or English flag. The samples were wrapped in moistened gazette paper and kept at 4 oC in a tightly closed nylon bag until they were processed.
Table 2 Number of samples collected in the crops under study
| Locality | Samples per crop | |||
|---|---|---|---|---|
| Total | ||||
| 1 | 28 | 36 | 16 | 80 |
| 2 | 80 | ND | ND | 80 |
| 3 | 12 | 24 | 44 | 80 |
| 4 | 18 | 26 | 36 | 80 |
| 5 | 80 | ND | ND | 80 |
| 6 | 32 | 15 | 33 | 80 |
| 7 | ND | 43 | 37 | 80 |
| 8 | ND | 26 | 54 | 80 |
| 9 | 38 | 26 | 16 | 80 |
| 10 | 80 | ND | ND | 80 |
| 11 | 27 | 53 | ND | 80 |
| 12 | 32 | 24 | 24 | 80 |
| 13 | 26 | 28 | 26 | 80 |
| 14 | ND | 80 | ND | 80 |
| Total | 453 | 381 | 386 | 1120 |
INDIRECT ELISA FOR THE DETECTION OF POTYVIRUS
An indirect ELISA was performed to determine the presence of Potyviruses in the samples, according to the manufacturer's instructions (Agdia, Inc., Indiana, USA). To do this, the available plant tissue was macerated in plastic bags, 300 mg of leaves, with 300 μL of indirect ELISA extraction buffer pH 7.4 (15 mM Na2CO3, 28.4 mM NaHCO3, 1 % PVP ) and centrifuged for 1 min at 7000 rpm. 100 μL of extract per well was added to the ELISA plate, covered with clear plastic and incubated at room temperature (RT) for 1 hour. After three washes with PBS-Tween pH 7.4 (0.02M NaHPO4, 0.08 M Na2HPO4, 0.15M NaCl, 0.003 M KCl and 10 % Tween-20), 98 μL of the antibody solution was added. Monoclonal anti-Poty (Agdia, Inc., Indiana, USA) per well, diluted 1: 200 in conjugate buffer (10X PBS, 10 % Tween-20, 2 % PVP) and incubated at 4 °C overnight. Subsequently, three washes were made with PBS-Tween, after which 95 μL per well of the solution of conjugated antibodies (anti-mouse conjugated with alkaline phosphatase) diluted 1: 200 in conjugate buffer and incubated for 1 h at TA was added. . Three more washes were carried out with PBS-Tween, and then 100 μL per well of the substrate solution was added: p-nitrophenol of concentration 1 mg L-1 in alkaline phosphatase substrate buffer with pH 9.8 (10 % diethanolamine, 0.5 mM MgCl2 6H2O, 3.8 mM NaN3). The plate was covered with aluminum foil and incubated at RT. Finally, spectrophotometric readings were taken at 405 nm after 30 min, 60 and 90 min of incubation. An ELISA reader LIUYI WD-2102A was used.
As positive controls to the infection by Potyvirus, the one supplied by the manufacturer (Agdia, Inc., Indiana, USA) in lyophilized form was used. Three samples of leaf tissue of Arabidopsis thaliana L. grown under controlled conditions in glass house were used as a negative control. Those samples whose absorbance readings at 405 nm had a minimum value of twice the reading obtained in the negative control were considered as positive 3.
The incidence of Potyvirus in the evaluated population was calculated by counting the number of plants positive to the indirect ELISA divided by the total of plants collected according to the methodology described by Gil et al. In 2011 12).
RESULTS
DETERMINATION OF THE INCIDENCE OF POTYVIRUS IN THE SAMPLED GEOGRAPHIC REGIONS
Among the samples collected were plants with typical Potyvirus symptoms such as mosaic, leaf wrinkling, internervial discoloration, yellowing and necrosis (Figure 1).

Figure 1 Symptomatic plants collected in the study showing wrinkling, mosaic, internervial discoloration, yellowing and necrosis. A) C. annuum. B) S. lycopersicum. C) N. tabacum
The results revealed the high incidence of Potyvirus in the areas evaluated. Between 34 and 75% of the plant material collected was positive by the Indirect ELISA test (D.O. 0.5-1.2) (Table 3). The highest values were recorded in locations three and nine located in the municipalities of San Juan and Martínez and San Antonio de los Baños, respectively; while the locality 14 located in the municipality Güira de Melena presented the lowest values (35%).
Table 3 Potyvirus incidence in the cultures sampled in the 14 included localities evaluated by the Indirect ELISA
| Locality |
|
|
|
Incidence of Potyvirus (%) |
|---|---|---|---|---|
| 1 | 43 | 64 | 63 | 56 |
| 2 | 43 | ND | ND | 42 |
| 3 | 58 | 58 | 73 | 66 |
| 4 | 61 | 58 | 42 | 51 |
| 5 | 54 | ND | ND | 53 |
| 6 | 34 | 67 | 58 | 38 |
| 7 | ND | 49 | 54 | 51 |
| 8 | ND | 46 | 65 | 59 |
| 9 | 61 | 69 | 75 | 66 |
| 10 | 64 | ND | ND | 64 |
| 11 | 44 | 45 | ND | 45 |
| 12 | 47 | 42 | 54 | 48 |
| 13 | 35 | 43 | 42 | 38 |
| 14 | ND | 35 | ND | 35 |
| Total | 50 | 49 | 48 | 51 |
ND- non-determined
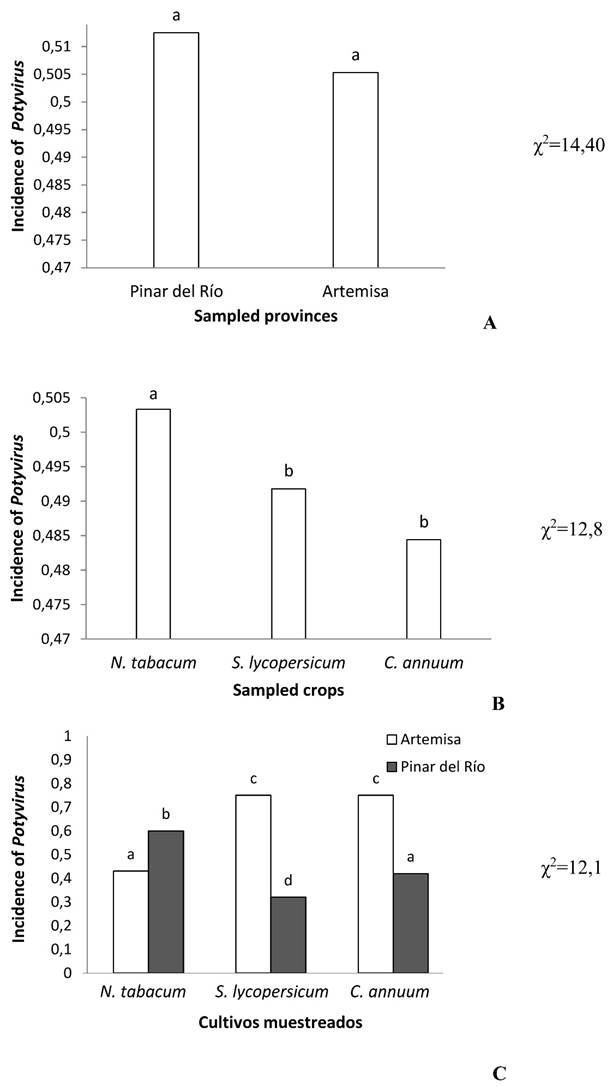
When making the general comparison between both provinces (Pinar del Río and Artemisa) no significant differences were detected regarding the incidence of Potyvirus for p≤0.05 (Figure 2A). The level of incidence in N. tabacum differs significantly from S. lycopersicum and C. annuum, while no differences were found between the values detected in S. lycopersicum and C. annuum for p≤0.05 (Figure 2B). The incidence of this viral genus for N tabacum in Pinar del Río is higher than in Artemisa, however for the other two crops it is higher in Artemisa. In turn, no significant differences were detected between S. lycopersicum and C. annuum in response to Potyvirus, both in Artemisa and in Pinar del Río (p≤0.05) (Figure 2C).
DISCUSSION
In Cuba, the presence of PVY and the tobacco etch virus (TEV) has been reported infecting N. tabacum and S. lycopersicum13. In C. annuum, the presence of pepper mottle virus (PepMoV) was also demonstrated 14. Likewise, some investigators found cytoplasmic inclusions characteristic of Potyviruses in cells from plants of C. annuum in Güira de Melena municipality of, Artemisa province 4. However, the quantification of the individual effects of these Potyviruses on the production of the evaluated crops has not been determined. In the country, there has been no monitoring of its incidence in Solanaceae, so it is not possible to analyze the evolution of this phenomenon in time and space.
In the present work, using immune-enzymatic techniques, it was determined that more than 50 % of the plants harvested are infected by Potyviruses (according to what is shown in Table III), an event that could be related to the use of sensitive cultivars and levels of infestation by aphids in the areas analyzed. The presence of susceptible cultivars would favor viral replication and, in turn, high levels of aphid infestation can contribute to their dissemination in the evaluated localities.
These results correspond to that reported, suggested that the members of the genus Potyvirus constitute one of the main problems in the production of various solanaceous crops in tropical regions with infection rates higher than 50 % 15. On the other hand, PVY has been identified in the Andean region of Colombia as one of the viruses most frequently affecting S. tuberosum, S. lycopersicum and C. annuum12,16,17, with at least three variants reported of PVY in the country 4.
The incidence of Potyvirus in the sampled areas indicates that this group is homogeneously distributed in the provinces of Artemisa and Pinar del Río, and in the three sampled crops, although its incidence in N. tabacum is higher than in S. lycopersicum and C. annuum, which could indicate that the circulating Potyvirus species in this region replicate more efficiently in N. tabacum. This result also shows that the type of host cultivated, exerts selective pressure on the viral species that predominates in a given environment and therefore influences its evolution.
Other researchers also found a high incidence of Potyviruses in different geographical locations, reporting that this viral group infected 75 % of the Solanaceae collected, and that 79 % of the positive plants were infected with PVY 18.
Many of the pathogenic species for N. tabacum and S. lycopersicum have the ability to infect S. tuberosum1. The type of transmission, mediated by aphids and by mechanical transmission, also favors the rapid mobility of the virus from an infected zone to a healthy area 18, which could indicate the presence of this viral group affecting S. tuberosum, in the region evaluated. However, this hypothesis must be demonstrated by including this crop in future studies
CONCLUSIONS
The genus Potyvirus is distributed homogeneously in the provinces of Artemisa and Pinar del Río and in the three sampled crops, although its incidence in N. tabacum is higher than in S. lycopersicum and C. annuum, which shows that the type of cultivated host exerts selective pressure on the viral species that predominates in a given environment and therefore influences its evolution.
BIBLIOGRAFÍA
1. Syller J, Grupa A. The effects of co-infection by different Potato virus Y (PVY) isolates on virus concentration in solanaceous hosts and efficiency of transmission. Plant Pathology. 2014;63(2):466-75. doi:10.1111/ppa.12095 [ Links ]
2. Cárdenas HCM, Sánchez PAG, Montoya MM. Detección del Potato virus Y (PVY) en tubérculos de papa mediante TAS-ELISA y QRT-PCR en Antioquia (Colombia). Bioagro. 2015;27(2):83-92. [ Links ]
3. Henao-Díaz E, Gutiérrez-Sánchez P, Marín-Montoya M. Análisis filogenético de aislamientos del Potato virus Y (PVY) obtenidos en cultivos de papa (Solanum tuberosum) y tomate de árbol (Solanum betaceum) en Colombia. Actualidades Biológicas. 2013;35(99):219-32. [ Links ]
4. Moodley V, Ibaba JD, Naidoo R, Gubba A. Full-genome analyses of a Potato virus Y (PVY) isolate infecting pepper (Capsicum annuum L.) in the Republic of South Africa. Virus Genes. 2014;49(3):466-76. doi:10.1007/s11262-014-1121-5 [ Links ]
5. Ivanov KI, Eskelin K, Lohmus A, Makinen K. Molecular and cellular mechanisms underlying Potyvirus infection. Journal of General Virology. 2014;95(Pt_7):1415-29. doi:10.1099/vir.0.064220-0 [ Links ]
6. Kamangar SB, Smagghe G, Maes M, De Jonghe K. Potato virus Y (PVY) strains in Belgian seed potatoes and first molecular detection of the N-Wi strain. Journal of Plant Diseases and Protection. 2014;121(1):10-9. doi:10.1007/BF03356485 [ Links ]
7. Karasev AV, Gray SM. Continuous and emerging challenges of Potato virus Y in Potato. Annual Review of Phytopathology. 2013;51(1):571-86. doi:10.1146/annurev-phyto-082712-102332 [ Links ]
8. Tsedaley B. A review paper on Potato virus Y (PVY) biology, economic importance and its managements. Journal of Biology, Agriculture and Healthcare. 2015;5(9):110-26. [ Links ]
9. González Arias G, Font C, Valdés Ramírez S. Diagnóstico de virus vegetales a nivel de grupo en el cultivo del pimiento (Capsicum annuum L.) mediante la técnica de microscopía óptica. Fitosanidad. 2002;6(3):3-7. [ Links ]
10. Rodríguez Y, Depestre T, Gómez O. Obtención de líneas de pimiento (Capsicum annuum) progenituras de híbridos F1, resistentes a enfermedades virales, a partir del estudio de cuatro sub-poblaciones. Ciencia e investigación agraria. 2007;34(3):237-42. doi:10.4067/S0718-16202007000300008 [ Links ]
10. Ricardo S, Díaz A, Acebo Y, Rives N, Almaguer M, Hernández A. Empleo del Sistema de Posicionamiento Global (GPS) en el manejo de ecosistemas agrícolas sostenibles. Revista Ciencias de la Tierra y el Espacio. 2010;6:21-31. [ Links ]
12. Torres JMC, Gil JF, Montoya MM. Incidencia de Potyvirus y caracterización molecular de PVY en regiones productoras de papa (Solanum tuberosum L.) de Colombia. Revista Colombiana de Biotecnología. 2011;13(1):85-93. [ Links ]
13. Crespo J, Domínguez M, Díaz A. Efecto de infecciones mixtas del Virus del Grabado del Tabaco (TEV) y Virus Y de la Papa (PVY) en variedades comerciales de tabaco negro en Cuba. Revista de Protección Vegetal. 2015;30(supl 1):59-59. [ Links ]
14. Quiñones M, Arana F, Alfenas-Zerbini P, Soto M, Ribeiro D, Diaz A, et al. First report of Pepper mottle virus in sweet pepper in Cuba. New Disease Reports. 2011;24:16. doi:10.5197/j.2044-0588.2011.024.016 [ Links ]
15. Mitiku A, Chala A, Beyene Y. The effect of intercropping of pepper with maize and sweet potato on infection of pepper (Capsicum annuumL.) by Potyviruse and yield of pepper in, Southern Ethiopia. International Journal of Technology Enhancements and Emerging Engineering Research. 2013;1(4):68-73. [ Links ]
16. Vásquez MA, Jaimes PG, Gutiérrez PA, Cotes JM, Montoya MM. Caracterización serológica y molecular de Potyvirus asociados a la virosis del tomate de árbol en Antioquia (Colombia). Acta Biológica Colombiana. 2010;15(3):145-63. [ Links ]
17. Villamil-Garzón A, Cuellar WJ, Guzmán-Barney M. Natural co-infection of Solanum tuberosum crops by the Potato yellow vein virus and Potyvirus in Colombia. Agronomía Colombiana. 2014;32(2):213-23. doi:10.15446/agron.colomb.v32n2.43968 [ Links ]
18. Arogundade O, Balogun OS, Akinyemi SOS, P LK. Surveys of virus diseases on pepper (Capsicum spp.) in South-west Nigeria. African Journal of Biotechnology. 2015;14(48):3198-205. doi:10.5897/AJB2015.14803 [ Links ]
Received: March 01, 2018; Accepted: January 24, 2019











 texto en
texto en