Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cultivos Tropicales
versión On-line ISSN 1819-4087
cultrop vol.40 no.1 La Habana ene.-mar. 2019
Original Article
Influence of the organic matter system and Azotobacter chroococcum over seedlings of coconut
1Centro de Desarrollo de la Montaña, Luz Caballero esquina 2 Sur, Guantánamo, Cuba
2Instituto de Suelos, Guantánamo, Cuba
3Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
4Instituto de Investigaciones Fundamentales en Agricultura Tropical “Alejandro de Humboldt” (INIFAT), calle 2 y 1, No. 17200, Santiago de Las Vegas, La Habana, Cuba
5Empresa Agropecuaria y Coco Baracoa, Guantánamo, Cuba
In Baracoa, municipality of the Guantánamo province the coconut seedling nurseries are being affected by the index low of germination and the little vigor of the seedling. The worm casting and the bacteria of the genus Azotobacter has been of those more employees in the seedling production, since promote the vegetable growth. The investigation over Randomized Block Design the combination of three levels of the substrates S:HL:FC (10:1:1), (4:1:1), (2:1:1) was studied with the inoculation of three strain of A. chroococcum (INIFAT-8, CDM-1, CDM-2) on the obtaining of seedling coconut belonging to the domesticated ecotipo "Indio Verde-1" in two soil, Haplic Arenosol (ARh) and Haplic Fluvic Gleysol (GFLh). As development indicators they were evaluated the index of speed germination of the seeds, the plant height (cm), the diameter in the stem base (cm) and the population of Azotobacter (UFC g-1 of soil). The possibility to diminish the organic mature levels employed in 50 % by means of the inoculation of strain of A. chroococcum isolated of the soil in study was demostrated. The combination soil: worm casting: fiber of Coconut (S:HL:FC) (4:1:1) with A. chroococcum CDM-1 in the soil ARh and A. chroococcum CDM-2 in the soil GFLh favored the increment of the studied variables, those that reached superior levels to which obtain higher levels to the recommended dose of mineral fertilization and to the organic mature.
Key words: Cocos nucifera; nursery; rhizobacteria; soil
INTRODUCTION
Coconut palm nucifera L is a perennial tropical crop of great uses, it grows in around 80 countries. In Cuba, the crop occupies approximately 13 186 ha with an average yield of 4.5 t ha-1 1, being of great importance the production of postures that guarantee the necessary material for the renovation and promotion of new areas. A diagnostic study carried out in the Mountain Development Center of Guantánamo province showed that nurseries have low germination rates, as well as weak positions that cause high mortality when they are moved to the field. This could be associated to different factors such as the use of seeds with immature embryos, together with limiting factors of the soil such as aeration, moisture retention and low fertility 2.
The use of an organic fertilizer system that takes into account the use of earthworm humus combined with biofertilizers would ensure favorable conditions for the germination and subsequent growth of plants 3. It is known that the addition of earthworm humus to soils and substrates, considerably increases the growth and productivity of a large number of crops, through the significant improvement in their physical, chemical and biological properties (4.
Plant growth promoting rhizobacteria (RPCV) are one of the biofertilizers widely used by direct and indirect mechanisms of growth promotion 5,6, with Azotobacter chroococcum being one of the most used bacterial nitrogen-fixing genera 7,8. The use of this species allows the shortening of the permanence period of the plants in the nurseries, and favors the increase in the morphological parameters of the plants 9, which makes them an alternative to evaluate in the growth of the postures of coconut tree in Cuba, since 1990, a program of production and application of A. chroococcum has been developed with selected strains, among which the strain INIFAT-8 10 stands out. The present investigation was developed with the objective of determining the influence of an organic fertilization system and inoculation of A. chroococcum on the germination of seeds and the growth of coconut tree positions in a conventional nursery.
MATERIALS AND METHODS
The experiment was carried out in the “Playa Duaba” and “Cabacú” nurseries, located in the municipality of Baracoa, Guantanamo province, on the Arenosol haplic (ARh) and Gleysol Fluvic, haplic (GFLh) soils, respectively 11. The experiment was repeated for two consecutive years, in which the combinations of three levels of the substrate were studied Soil: Earthworm Humus: Coconut Fiber (S: HL: FC) (10: 1: 1, 4: 1: 1, 2: 1: 1, v/v/v) 12) with three strains of A. chroococcum (CDM-1, CDM-2 and INIFAT-8).
The strains of A. chroococcum, came from the collection of the National Institute of Fundamental Investigations of Tropical Agriculture “Alejandro de Humboldt” (INIFAT). Strains CDM-1 and CDM-2 were isolated from the soils present in the “Playa Duaba” and “Cabacú” nurseries, respectively. The bioproducts made had a concentration of 9 x 1010 CFU per g of support 13 and were applied by direct spray to the soil and the seed at a dose of 1 kg ha-1 14.
Three controls constituted by mineral fertilization (NPK 100 %) were also used, for which the complete formula 9:13:17 was used at a rate of 45 g per seed fractioned to 33 % at 30 days after sowing (das) and the rest at 90 das 15, the substrate S: H: FC 1: 1: 1 v/v/v and the soil. In all the cases for the elaboration of the substrate, the soil present in the nursery was used.
The seeds were obtained from healthy mother plants, belonging to the coconut domesticated ecotype "Indio Verde-1" 16. A randomized block design with bifactorial arrangement (3x3) and three replications was used. Each experimental plot within the block contained 20 seeds sown at a distance of 0.05 x 0.20 m.
The experiment lasted 180 days. At 120 das the germination rate index (IVG) was calculated by the formula
The data of the variable counting populations of A. chroococcum in the rhizosphere were transformed by the formula log (x). The results for all the variables evaluated showed a similar behavior in the two years studied, so the data corresponding to the average of the two years were analyzed. The factorial variance analysis and the Duncan Multiple Range Test (p(0,05) were used for the statistical processing of the treatments. To compare the controls with each of the treatments, a double classification ANOVA was performed, the EE was determined, with which the confidence interval (CI) was calculated for the controls with a level of significance of 95 % and verified if the means of each treatment were contained within the confidence interval. The statistical package STATGRAPHIC version 15.2 was used.
RESULTS AND DISCUSSION
The analysis carried out showed interaction of the factors in the two soils under study, for all the variables evaluated, except for the IVG. No effect of the levels of organic fertilizer on the IVG was found, not being so for the strains factor of A. chroococcum, which did have significance.
It was observed that in the soil ARh the strains isolated from the studied soils (CDM-1 and CDM-2) showed no differences between them and were superior to the strain INIFAT-8 and to the controls, with average values between 0.50 and 0,52, while in the GFLh soil, the best values corresponded to strain CDM-2, with a value of 0.42 (Figure 1). In the treatments inoculated with both strains, no significant differences were observed between the three levels of the organic fertilizers and the control S: HL: FC (1: 1: 1).
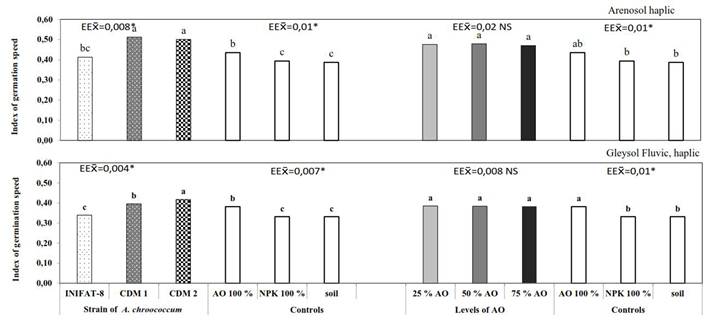
Figure 1 Index of germination speed of coconut seeds ecotype Indio Verde-1 up to 120 days after sowing, subjected to the combined treatments of three strains of A. chroococcum and three levels of organic fertilizer on the soils Arenosol haplic and Gleysol Fluvic
With respect to the control S: HL: FC (1: 1: 1), in the ARh soil the treatment where the strain CDM-1 was used with S: H: FC (4: 1: 1) showed a reduction in four days the start of germination, while in the GFL soil it decreased in 12.8 days (data not shown).
In the stem height variable, the best behavior in the soil ARh was observed, when the strain CDM-1 was used with S: HL: FC (4: 1: 1), which differed from the other treatments and controls of reference (Figure 2A). In general, the variants where the strains of A. chroococcum were inoculated combined with the three levels of the organic fertilizers, were higher when compared with the soil control.
When analyzing the results in the GFLh soil, it was found that when combining A. chroococcum with the three levels of organic fertilizer, the CDM-2 strain influenced the obtaining of the best values for the height at the base of the stem (Figure 2C), followed by CDM-1 and by INIFAT-8, with which the lowest values were obtained. The strain CDM-2 combined with S: HL: FC (4: 1: 1) favored the best response for this variable, unlike the rest of the treatments and the controls.
The analysis of the stem diameter in the ARh soil showed the best results when the strain CDM-1 and S: HL: FC (4: 1: 1) were combined, with difference from the other treatments and controls. On the other hand, the treatments where CDM-2 was used with the three levels of organic fertilizer showed similarity when compared with the treatments inoculated with the INIFAT-8 strain. It was also obtained that these last mentioned variants showed similarity with the S: HL: FC control (1: 1: 1) and differences with the 100% NPK and soil controls (Figure 2B).
For the treatments performed with the GFLh soil (Figure 2D) the best results were obtained, with the combination of the strain CDM-2 and the three levels of organic fertilizer, which showed differences with the controls.
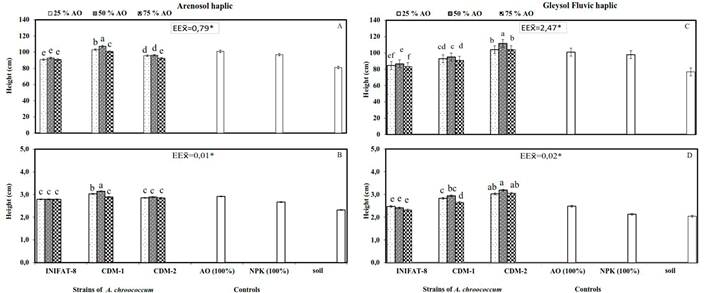
Figure 2 Height and diameter at the base of the shoot 180 days after sowing of coconut plants ecotype Indio Verde-1, subjected to the combined treatments of three strains of A. chroococcum and three levels of organic fertilizer on the Arenosol and haplic soils. Gleysol Fluvic, haplic
In the analysis of the quantification of Azotobacter populations in the soil ARh (Figure 3A) the best results were found in the treatment where the strain A. chroococcum CDM-1 and S: HL: FC (4: 1: 1) was combined with significant differences with respect to the other treatments and controls. On the other hand, in the variants where the GFLh soil was used in the preparation of the substrate, the counting of the populations of Azotobacter in the rhizosphere (Figure 3B) showed the best responses with the inoculation of the strain A. chroococcum CDM-2 combined with the levels of S: HL: FC 10: 1: 1 and 4: 1: 1, with significant differences from the rest of the treatments and controls. In both soils the lowest levels were reached with the INIFAT-8 strain and the soil control.
The lack of response in the treatment formed by the soil without application of Azotobacter is possibly related to the limiting factors of these soils, as well as the low populations of Azotobacter present in them.
The low percentages of moisture retention in the Arenosol haplic soil could hinder the flow of masses and the diffusion of nutrients, limiting the absorption of nutrients by the plant 20. On the other hand, the decrease of oxygen levels in the GLFh soil due to its gleyic properties 11, could have prevented the normal development of the roots, and with it a low absorption of water and nutrients. These properties could have been improved with the use of organic fertilization since it allows the formation of soil aggregates, which improve the balance between macros and micropores and with it the retention of water, in addition to the increase of organic matter levels and the state of soil fertility 21.
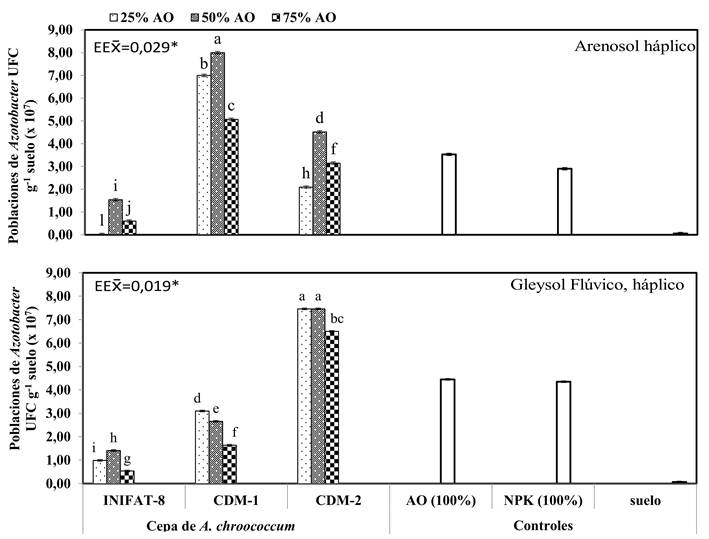
Figure 3 Quantification at 180 days after sowing of Azotobacter populations in the rhizosphere of ecotype Indio Verde-1 coconut poses submitted to the combined treatments of three strains of A. chroococcum and three levels of organic fertilizer on the soils Arenosol haplic and Gleysol Fluvic, haplic.
The studies presented provide evidence that the strains of Azotobacter used responded most effectively in the soils from which they were isolated. The best response was found with the use of S: HL: FC (4:1:1) when combined with the strain CDM-1 in the ARh and with the CDM-2 strain in the GFLh, which makes it possible to reduce the levels of organic fertilizer employees by 50 %, by using this rhizobacteria.
It has been found that at the concentrations of these bacteria present in Cuban soils (104-105 CFU g-1 rhizospheric soil), it is difficult to observe responses in the growth of plants, so the inoculation of them allows increasing the existing levels (up to 109 CFU g-1 of rhizospheric soil) favoring a positive response of crops 13.
The autochthonous strains, being better adapted to the conditions studied, were able to function more quickly and effectively than the strain INIFAT-8, which apparently could find antagonism with resident microbial populations in these soils. It is proposed that when free-living bacteria are inoculated, the variability in the responses found is subject to different conditions, among which are the presence of resident microbial communities of the rhizosphere, with which interrelationships of synergism and antagonism are established 22.
The response observed for the different levels of organic fertilization was given by the fact that being Azotobacter a heterotrophic bacterium, it uses several organic sources of energy, which include hemicellulose, starch, sugars, alcohols and organic acids, which come from organic matter present in soils 23. However, the activities of this bacterial group in its relationship with the plant will be favored by the low levels of nitrogen in the soil, which favors the fixation of this element. On the other hand, its effectiveness decreases as the soils have a higher content of organic matter. When biofertilizer is applied, it must be fertilized with organic amendments to avoid soil impoverishment 15.
The positive results observed in the stimulation of seed germination and in the growth of coconut tree postures could be associated to the application form of the inoculum of this biofertilizer. It was applied by direct spraying to the seed at the time of sowing, which could favor the hydration of the mesocarp and endocarp, and in this way achieve that optimum moisture was reached more quickly for the embryo to initiate germination.
This effect, together with the ability of these bacteria to produce growth regulating substances 24,25, caused them to penetrate the seminal cortex, accelerate germination and root development. In the genome of some strains of rhizobacteria isolated from the rhizosphere of the coconut tree, the H2S gene has been identified, which is involved in the synthesis of hydrogen sulfide, a compound linked to the increase in seed germination 26.
Another proposed mechanism through which Azotobacter may have promoted plant growth is the production of the enzyme 1-amino-cyclopropane-1-carboxylate deaminase (ACC deaminase), which, in addition to preventing the occurrence of diseases 27, it can facilitate plant growth and favor the initiation of embryo growth, together with the synthesis of indole acetic acid (AIA) and the regulation of ethylene levels 24.
Different authors suggest the importance of ethylene in the germination process 26,28-30, in the decrease of dormancy and the emission of the radicle in different species 28-30. Ethylene production begins immediately after imbibition and increases with time during germination 22.
Strains of rhizobacteria isolated from coconut culture have been shown to produce AIA, gibberellic acid, ammonium, siderophores, proteases, catalases, cellulases, solubilization of phosphates 29, N2 fixation 31 as well as protection against different biotic 32-35 and abiotic 36,37 stresses are the mechanisms by which these bacteria promote plant growth, which places them as potential for the production of bio-inoculants in the organic management of this crop 26,30.
CONCLUSIONS
It was shown that it is possible to reduce the levels of organic fertilization employed by 50 % by inoculating strains of Azotobacter chroococcum isolated from the soils under study.
The combination Soil: Earthworm Humus: Coconut Fiber (S: HL: FC) (4: 1: 1) with A. chroococcum CDM-1 in the soil ARh and A. chroococcum CDM-2 in the soil GFLh favored the increase in the variables studied, which reached levels higher than those obtained with the recommended dose of mineral fertilization and organic fertilization.
BIBLIOGRAFÍA
1. FAOSTAT. Agricultural data [Internet]. FAO, Roma; 00/09/2018. Available from: http://www.fao.org/faostat/en/#home1. [ Links ]
2. Blanco AI. Influencia de las características de la semilla, el riego y la fertilización orgánica en la calidad de las posturas de cocotero (Cocos nucifera L.). [Tesis de Maestría]. [Granma]: Universidad de Granma; 2007. 87 p. [ Links ]
3. Lok S, Suárez Y. Efecto de la aplicación de fertilizantes en la producción de biomasa de Moringa oleifera y en algunos indicadores del suelo durante el establecimiento. Revista Cubana de Ciencia Agrícola. 2014;48(4):399-403. [ Links ]
4. Broz AP, Verma PO, Appel C. Nitrogen dynamics of vermicompost use in sustainable agriculture. Journal of Soil Science and Environmental Management [Internet]. 2016;7(11):173-83. doi:10.5897/JSSEM2016.0587 [ Links ]
5. Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability-A Review. Molecules. 2016;21(5):573. doi:10.3390/molecules21050573 [ Links ]
6. Himaman W, Thamchaipenet A, Pathom-aree W, Duangmal K. Actinomycetes from Eucalyptus and their biological activities for controlling Eucalyptus leaf and shoot blight. Microbiological Research. 2016;188-189:42-52. doi:10.1016/j.micres.2016.04.011 [ Links ]
7. Romero-Perdomo F, Abril J, Camelo M, Moreno-Galván A, Pastrana I, Rojas-Tapias D, et al. Azotobacter chroococcum as a potentially useful bacterial biofertilizer for cotton (Gossypium hirsutum): Effect in reducing N fertilization. Revista Argentina de Microbiología. 2017;49(4):377-83. doi:10.1016/j.ram.2017.04.006 [ Links ]
8. Beltrán ME. La solubilización de fosfatos como estrategia microbiana para promover el crecimiento vegetal. Revista Corpoica: Ciencia y Tecnología Agropecuaria. 2014;15(1):101-13. [ Links ]
9. León Y, Hernández JM, Rodríguez N, Martínez R. Aplicación de Azotobacter chroococcum en la producción de plántulas de tabaco negro. Cultivos Tropicales. 2012;33(2):29-32. [ Links ]
10. Martínez VR, Dibut AB. Biofertilizantes bacterianos. La Habana, Cuba: Científico Técnica; 2012. 279 p. [ Links ]
11. Hernández A, Pérez J, Bosch D, Castro N. Clasificación de los suelos de Cuba. Mayabeque, Cuba: Ediciones INCA; 2015. 93 p. [ Links ]
12. MINAG, ACTAF, IIFT. Instructivo técnico para el cultivo del coco [Internet]. 1st ed. La Habana, Cuba: Instituto de Investigaciones en Fruticultura Tropical (IIFT); 2011. 15 p. Available from: http://www.actaf.co.cu/index.php?option=com_mtree&task=att_download&link_id=501&cf_id=2412. [ Links ]
13. Martínez VR, Dibut B. Practical aplications of bacterial biofertilizers and bioestimulators. In: Biological approaches to sustainable soil systems [Internet]. New York: CRC/Taylor & Francis; 2006 [cited 07/01/2019]. p. 467-77. Available from: https://books.google.com.cu/books?id=lgbOBQAAQBAJ&pg=PA475&lpg=PA475&dq=Practical+applications+of+bacterial+biofertilizers+and+biostimulators&source=bl&ots=iIxnJ4st__&sig=fXJukP382VM6Jz3AhHlN9AlNmaE&hl=es&sa=X&ved=2ahUKEwjfzJG_j9zfAhVBiFkKHSfOCWMQ6AEwAXoE13. [ Links ]
14. Dibut B. Biofertilizantes como insumos en agricultura sostenible. La Habana, Cuba: Editorial Universitaria; 2009. 113 p. [ Links ]
15. Ohler JG. Modern coconut management: palm cultivation and products [Internet]. London, England: Intermediate Technology Pub.; 1999. 458 p. Available from: http://agris.fao.org/agris-search/search.do?recordID=XF200039140915. [ Links ]
16. Alonso M, Cueto JR, Santos Y, Romero W, LLauger R, Rohde W. Variabilidad morfológica y molecular de una población de cocoteros verdes en la región de Baracoa. Cultivos Tropicales. 2007;28(3):69-75. [ Links ]
17. Valfré-Giorello T, Ashworth L, Renison D. Patrones de germinación de semillas de Sebastiania commersoniana (Baillon) Smith & Downs (Euphorbiaceae), árbol nativo del Chaco Serrano de interés en restauración. Ecología austral. 2012;22(2):92-100. [ Links ]
18. Peries R, Everard J. River sand as an alternative to top soil for raising coconut seedlings in polybags. COCOS. 1993;9:40-6. [ Links ]
19. Aquilanti L, Favilli F, Clementi F. Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil Biology and Biochemistry. 2004;36(9):1475-83. doi:10.1016/J.SOILBIO.2004.04.024 [ Links ]
20. Borda-Molina D, Pardo-García JM, Montaña-Lara JS, Martínez-Salgado MM. Influencia de la materia orgánica y Azotobacter nigricans en un cultivo de Stevia rebaudiana B. Universitas Scientiarum. 2011;16(3):282-93. [ Links ]
21. Duartel L, Horst C, Manfio CE, Yoshimitsu S, Prieto HE, Bruckner CH, et al. Substrate, lime, phosphorus and top dress fertilization in macaw palm seedling production. Revista Árvore. 2016;40(2):235-44. doi:10.1590/0100-67622016000200006 [ Links ]
22. Valery A, Reyes I. Evaluación de rizobacterias promotoras del crecimiento bajo diferentes esquemas de fertilización en el cultivo de maíz variedad HIMECA-95. Revista Colombiana de Biotecnología. 2013;15(2):81-8. [ Links ]
23. Rodriguez M, Rivas F. Dinámica poblacional de Azotobacter spp., en relación al contenido de materia orgánica en una plantación de E. grandis Hill - Purumayo, Oxapampa - Pasco. Ambiente. 2017;1(1-2):38-47. [ Links ]
24. Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiological Research. 2014;169(1):30-9. doi:10.1016/J.MICRES.2013.09.009 [ Links ]
25. Hussain A, Srinivas P. Evaluation of plant growth promoting traits by Pseudomonas and Azotobacter isolated from Rhizotic Soils of two selected agroforestry tree species of Godavari Belt Region , India . 2013;4(3):431-6. [ Links ]
26. Gupta A, Gopal M, Thomas G V., Manikandan V, Gajewski J, Thomas G, et al. Whole genome sequencing and analysis of plant growth promoting bacteria isolated from the rhizosphere of plantation crops coconut, cocoa and arecanut. Pellegrini M, editor. PLoS ONE. 2014;9(8):1-14. doi:10.1371/journal.pone.0104259 [ Links ]
27. García-Fraile P, Menéndez E, Rivas R. Role of bacterial biofertilizers in agriculture and forestry. AIMS Bioengineering. 2015; 2(3):183-205. doi:10.3934/bioeng.2015.3.183 [ Links ]
28. El-Maarouf-Bouteau H, Sajjad Y, Bazin J, Langlade N, Cristescu SM, Balzergue S, et al. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant, Cell & Environment. 2015;38(2):364-74. doi:10.1111/pce.12371 [ Links ]
29. Ortega M, Shagarodsky T, Dibut B, Ríos Y, Tejeda G, Gómez L. Influencia de la interacción entre el cultivo del garbanzo (Cicer arietinum29. L.) y la inoculación con cepas seleccionadas de Mesorhizobium29. spp. Cultivos Tropicales. 2016;37(5):20-7. [ Links ]
30. Sakthivel K, Subramani T, Manigundan K, Velmurugan A, Gautam RK. Characterization of coconut rhizobacteria for plant growth promoting traits. Journal Andaman Sciences Association. 2015;20(2):136-40. [ Links ]
31. Sahoo RK, Ansari MW, Dangar TK, Mohanty S, Tuteja N. Phenotypic and molecular characterisation of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma. 2014;251(3):511-23. doi:10.1007/s00709-013-0547-2 [ Links ]
32. Villa JA, Ray EE, Barney BM. Azotobacter vinelandii siderophore can provide nitrogen to support the culture of the green algae Neochloris oleoabundans and Scenedesmus sp. BA032. FEMS Microbiology Letters. 2014;351(1):70-7. doi:10.1111/1574-6968.12347 [ Links ]
33. Khaitov B, Patiño-Ruiz JD, Pina T, Schausberger P. Interrelated effects of mycorrhiza and free-living nitrogen fixers cascade up to aboveground herbivores. Ecology and Evolution. 2015;5(17):3756-68. doi:10.1002/ece3.1654 [ Links ]
34. Chennappa G, Naik M, Adkar-Purushothama C, Amaresh Y, Sreenivasa M. PGP potential, abiotic stress tolerance and antifungal activity of Azotobacter strains isolated from paddy soils. Indian Journal of Experimental Biology. 2016;54(5):322-31. [ Links ]
35. Baars O, Zhang X, Gibson MI, Stone AT, Morel FMM, Seyedsayamdost MR. Crochelins: Siderophores with an Unprecedented Iron-Chelating Moiety from the Nitrogen-Fixing Bacterium Azotobacter chroococcum. Angewandte Chemie. 2018;130(2):545-50. doi:10.1002/ange.201709720 [ Links ]
36. Omer A, Emara H, Zaghloul R, Abdel M, Dawwam G. Potential of Azotobacter salinestris as plant growth promoting rhizobacteria under saline stress conditions. Research Journal Of Pharmaceutical Biological And Chemical Sciences. 2016;7(6):2572-83. [ Links ]
37. Rizvi A, Khan MS. Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicology and Environmental Safety. 2018;157:9-20. doi:10.1016/J.ECOENV.2018.03.063 [ Links ]
Received: July 09, 2018; Accepted: October 09, 2018











 texto en
texto en 


