Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cultivos Tropicales
versión On-line ISSN 1819-4087
cultrop vol.41 no.1 La Habana ene.-mar. 2020 Epub 01-Mar-2020
Original Article
Chemical stability and biological activity of QuitoMax® during storage
1Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
2Unidad Científico Tecnológica de Base "Los Palacios". Km 1½ carretera La Francia, Los Palacios, Pinar del Río, Cuba. CP 22900
3Instituto de Ciencia y Tecnología de Materiales, Laboratorio de Polímeros. Calle Zapata s/n, entre G y Carlitos Aguirre, Vedado, La Habana, Cuba. CP 10400
QuitoMax® is a biologically active, biocompatible and biodegradable product based on chitosan. It has the ability to stimulate plant growth, yield and induce defensive mechanisms against pathogens. Regardless of its field of action, this product must be able to maintain its chemical characteristics during storage. The objectives of this work were to evaluate the chemical characteristics of QuitoMax® during storage and biological activity in tomato seedlings. The conductivity, pH and mass of soluble chitosan were determined as chemical characteristics. Seeds previously treated with QuitoMax® were evaluated for germination and morphoagronomic variables of tomato postures 25 days after planting. As results, there were no significant changes in conductivity, pH and mass of soluble chitosan in the period evaluated. Likewise, no differences were between the conserved product found and one newly produced when evaluating the germination and plants height. The results obtained show that the product maintains the chemical characteristics and biological activity at 270 days of conservation.
Key words: chitin; conductivity; polymer; chitosan
INTRODUCTION
The use of bioproducts in agriculture as an alternative to the indiscriminate use of fertilizers and pesticides has gained popularity in recent years. These products are biodegradable, non-toxic and are capable of acting on the physiology of plants promoting increases in plant growth and development 1. Chitosan is part of these bioproducts and is obtained from chitin, which is the second most abundant polymer in nature after cellulose. It is a copolymer composed of 2-acetamido-2-deoxy-D-glucose and 2-amino-2-deoxy-D-glucose units linked together by β (1→4) glycosidic bonds, with a predominance of the latter. It is obtained from by-products from the shellfish industry although it can be found in nature in the cell wall of some fungi 2. From a biological point of view, this polymer has been shown to have great biological activity regardless of its molecular mass 3. At the proper concentration, it is capable of advancing germination, promoting plant growth and development, as well as inducing defensive and resistance mechanisms against pathogens 1,4,5. Its antibacterial activity against Pectobacterium carotovorum has been with the use of thyme essential oils proven encapsulated in chitosan nanoparticles 6. Also in the form of films for the coating of fruits with the aim of extending the shelf life in post-harvest 7-9.
Chitosan is widely used in waste management, food processing, nanotechnology, medicine, biotechnology, and agriculture. It is a very interesting material in pharmaceutical applications due to its low toxicity, biodegravability and biocompatibility 7. It is a natural polycation with antifungal activity 10 with the capacity to induce resistance to pathogen attack 11. The characterization of this polymer is based on the determination of its structure by different methods such as Scanning Electron Microscopy (SEM), Energy Dispersed Spectroscopy (EDS), Fourier Transform Infrared Spectroscopy (FTIR) and Z Potential among others 6. Also by determining its molecular mass by viscosimetry 12, gel filtration chromatography, and the degree of deacetylation by potentiometry or spectroscopic methods 13, as well as the quantification of the ash content and the humidity present in it 14.
However, once these characteristics are known and its dilution has been carried out, conductivity, pH and concentration are parameters that can be taken into account since they provide information on the behavior of this polymer in solution. This is due to the fact that the conductivity determination allows predicting changes in the structure of this polysaccharide in solution since the chitosan in acidic conditions is a polyelectrolyte whose charge parameter λ is proportional to the linear charge density that depends on the pH and the degree of deacetylation 12. The control of the pH is important because the properties of the chitosan in solution are given by the high content of the primary amino groups (NH3) with a pKa of 6.3. The positive charge of the NH3 group at low pH values converts chitosan into a water soluble cationic polyelectrolyte. When the pH increases above 6.0 the positive charge of the NH3 group is lost and the chitosan becomes insoluble. The transition of chitosan from soluble to insoluble occurs in a pH range 6.0-6.5 in the pKa of the primary amino groups, which depends closely on the degree of deacetylation and the N-deacetylation method 15,16. The mass of soluble chitosan can provide information on the concentration of soluble chitosan present in the product.
The National Institute of Agricultural Sciences has a technology to obtain a chitosan-based product named QuitoMax®. This product has been evaluated and extended in various crops of economic importance such as potatoes (Solanum tuberosum L.), cucumbers (Cucumis sativus L.), peppers (Capsicum annuum L.), beans (Phaseolus vulgaris L.), soybeans (Glicine max L.), corn (Zea mays L.), rice (Oryza sativa L), and tobacco (Nicotiana tabacum L.). The productive increases generated from the application of QuitoMax® have increased the demand for this product.
The tomato (Solanum lycopersicum L.), is one of the vegetables that is most produced and industrialized worldwide. In Cuba, this crop represents 50 % of the total area dedicated to vegetables and its production ranges around 750,000 t 1. The studies of application of the QuitoMax® in tomato reveal a stimulus in the growth and yield variables of this crop 1.
Taking into account the previously exposed background, the objective of this work was to evaluate the chemical characteristics and the biological activity of this product during its storage. Among the chemical characteristics to be determined are the conductivity, the pH and the mass of soluble chitosan; while the biological activity will be evaluated through the effect on the germination of tomato seeds and the height of their positions.
MATERIALS AND METHODS
The research was carried out in the Biostimulants production laboratory and in the light room belonging to the Department of Plant Physiology and Biochemistry of the National Institute of Agricultural Sciences. It is located in San José de las Lajas municipality, Mayabeque province, Cuba.
Three batches of QuitoMax® were prepared at a concentration of 4 g L-1 containing sodium benzoate at 0.5 g L-1 at pH 4.5. The chitosan used in production has a molecular mass of 106 KDa and a degree of deacetylation (GD) of 73 % determined by potentiometric titration, 13 % humidity and 2.2 % ash. QuitoMax® was stored in 240 ml bottles at 30 ºC. The analysis period was 30, 60, 90, 180 and 270 days.
The conductivity was determined at the dilutions of QuitoMax® at 0.5 g L-1 concentration, with the use of a CRISON ECMeter 30+ conductivity meter manufactured by Crison Instrumenst SA in the United States previously calibrated with standard solutions of 147 μS cm -1, 1413 μS cm-1 and 12.88 ms cm-1 a 25 ºC.
The pH was determined with a QuitoMax® solution of 4g L-1 concentration, using an Inolab pH 720 pH meter made in Germany, previously calibrated with pH 4.01 and 7.00 standard solutions.
The mass of soluble chitosan was determined by previously filtering the QuitoMax® with a number 1 frit, 80ml of the product were taken and the pH was adjusted to 10 to achieve the precipitation of the chitosan present in the solution. The solid was separated from the liquid by filtration with pre-weighed filter paper on a Sartorius TE214S analytical balance made in Germany. The solid next to the filter paper was dried in a Binder ED115 stove made in Germany, until constant weight. The mass of soluble chitosan was determined from the following equation..
Where pF is the weight in grams of the filter paper, m is the weight in grams of the precipitated chitosan and 0.265 is the theoretical mass of chitosan-determined taking into account the humidity of the chitosan and the ash content. The mass of soluble chitosan was expressed as a percentage (%). All determinations were made in triplicate.
Additionally, the germination evaluation was carried out 270 days after the product was stored. The seeds were soaked for 1 hour in a QuitoMax® solution of 1 g L-1 concentration, and then they were dried and dispersed in Petris plates at a rate of 50 seeds per plate for each treatment including the control. The distilled water was taken as control, treatment 1 corresponded to a recently produced QuitoMax® and treatment 2 is the preserved QuitoMax® from lot 2. The seeds were germinated in a growth chamber WTW TS606/3-ia 28 ºC and evaluations are made at 24, 48, 72 hours.
Subsequently, the pre-germinated seeds were sown in containers of 6.6 cm in diameter and 9.7 cm in height on Ferralitic Red Compacted Eutric soil, according to the Cuban Soil Classification 17. Posture growth took place in the light room at a temperature of 27 ºC and 36 % relative humidity, with a photoperiod of 16 light hours and 8 hours of darkness. At 25 days after planting, the height of the plant was evaluated at 15 positions. The height of the postures (cm) was measured with a graduated ruler, from the root neck to the axilla of the youngest leaf.
The data were processed by means of analysis of variance of simple ANOVA classification and the means were compared by the Tukey test (p≤0.05). For statistical analyzes, the STATGRAPHIC PLUS statistical package was used.
RESULTS AND DISCUSSION
The determination of the pH in the three batches showed that this indicator remained stable throughout the evaluated period, between 4.45-4.57 (Figure 1), consistent with the values reported in the product record of 4.5 ± 0,1. The stability of the pH in this range is necessary since a high increase could lead to a decrease in the action of sodium benzoate as an antimicrobial, taking into account that it is only effective in acidic conditions. The inhibition concentrations of the microorganisms are around 0.05-0.1 % of the undissociated acid, mainly in acidic foods (pH less than or equal to 4-4.5) 18. So the pH at which the product is kept guarantees the solubility of chitosan and the antimicrobial properties of sodium benzoate.
 The bars on the mean values represent the confidence interval of the means α = 0.5
The bars on the mean values represent the confidence interval of the means α = 0.5Figure 1 pH records in three batches of QuitoMax® during 270 days of storage
On the other hand, the conductivity study in the three batches maintained stability in the storage period (Figure 2) and the differences between batches 1, 2 and 3 are attributable to the product preparation process. The conductivity of this product is given by the contribution of all the species present in it, from chitosan, sodium benzoate and sodium hydroxide used to adjust the pH. Therefore, variations can come from the heterogeneity of the chitosan to the amounts of sodium hydroxide used in the pH adjustment. The stability observed over time indicates that there was no variation in the solubility of chitosan in the storage period. Conductivity measurements can show the evolution of protonation, with a progressive increase due to the gradual solubilization of chitosan. Complete solubilization is obtained when the degree of dissociation α ≥ 0.5 and the stoichiometric relationship between acetic acid and chitosan [TeX:] <math> <mrow> <mrow><mrow> <mrow><mo>[</mo> <mrow> <mi>A</mi><mi>c</mi><mi>O</mi><mi>H</mi></mrow> <mo>]</mo></mrow></mrow><mo>/</mo><mrow> <mrow><mo>[</mo> <mrow> <mi>C</mi><mi>h</mi><mi>i</mi><mi>t</mi><mo>−</mo><mi>N</mi><msub> <mi>H</mi> <mn>2</mn> </msub> </mrow> <mo>]</mo></mrow></mrow></mrow> </mrow> </math> is 0.6 19. Conductivity as an analysis tool has also been used in the study of the formation of polyelectrolyte complexes between chitosan and pectin, in which an increase in it is observed as the polyelectrolyte complex is formed, which allows determining the stoichiometric relationship between these two polysaccharides 20.
The little variability of conductivity over time determined in this study coincides with conductivity studies carried out by other authors at different concentrations of chitosan and acetic acid. In these, it is referred that the conductivity in the chitosan solutions does not undergo changes during its storage and that the variations are consequences of measurement errors 15.
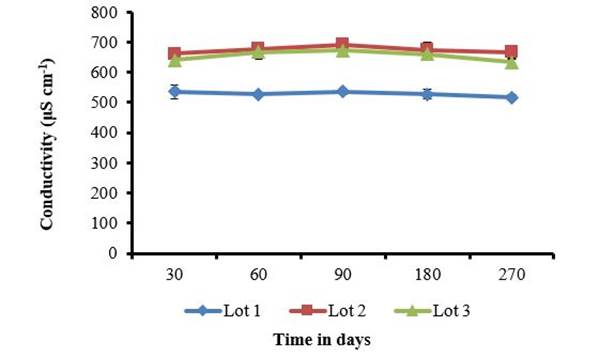 The bars on the mean values represent the confidence interval of the means α = 0.5
The bars on the mean values represent the confidence interval of the means α = 0.5Figure 2 Conductivity records in three batches of QuitoMax® during 270 days of storage
On the other hand, when evaluating the solubility of chitosan in general, the insoluble material present in 1 % acetic acid solutions is determined at a temperature of 25 ºC 21. However, in this work the mass of soluble chitosan present in the QuitoMax® at 30 ºC was evaluated, as an indicator of the concentration of chitosan dissolved in the product (Figure 3). In general, in the three batches the mass of dissolved chitosan was found to be over 80 %, with no differences between the same batches in the evaluated periods.
 The bars on the mean values represent the confidence interval of the means α = 0.5
The bars on the mean values represent the confidence interval of the means α = 0.5Figure 3 Records of the mass of soluble chitosan (MQS) in three batches of QuitoMax® during 270 days of storage
As for the biological activity, when evaluating the germination in tomato seeds of cultivar "Amalia" treated with the product, it was observed that after 270 days of conservation there were no significant differences p <0.05 between the preserved product and the fresh and if with the control (Figure 4). After 72 hours, there were no significant differences between any of the treatments. In general, after 270 days of conservation, a positive effect on germination was observed at 48 hours. This increase in germination may be because chitosan has excellent film-forming properties, which facilitates the formation of a semi-permeable film on the surface of the seed and helps to maintain moisture and promote germination 22. Although the mechanism by which chitosan exerts an effect on germination is still unknown, a positive effect has been reported in corn 23 and wheat 22 seeds.
Las barras sobre los valores medios representan el intervalo de confianza de las medias α =0,5.
 Equal letters do not differ statistically according to the Tukey HSD Multiple Range Test
Equal letters do not differ statistically according to the Tukey HSD Multiple Range TestFigure 4 Effect of fresh QuitoMax® (T1) and preserved (T2) on tomato seed germination
The evaluation of the height of the laying at 25 days after sprouting did not show significant differences between the preserved product and the fresh one, but with the control (Figure 5). This behavior was similar to that observed in germination at 48 hours. Although the mechanism by which chitosan promotes posture growth has also not been published, the positive effects on growth and development of various crops have been widely documented in rice 24, tomato 1, potato 5, soy 3 among others.
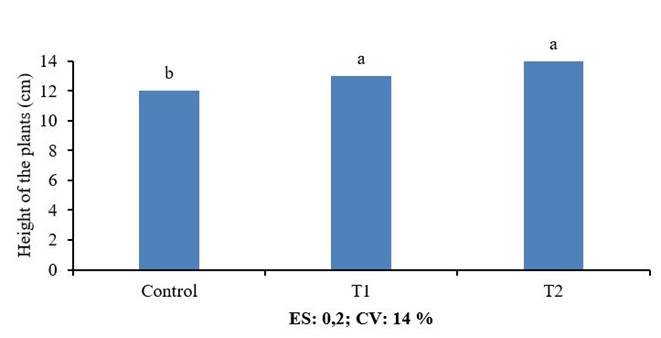 Equal letters do not differ statistically according to the Tukey HSD Multiple Range Test
Equal letters do not differ statistically according to the Tukey HSD Multiple Range TestFigure 5 Effect of fresh (T1) and preserved (T2) QuitoMax® on posture height
The stimulation of plant growth and development by chitosan has been associated with physiological processes. One of these processes is to avoid the loss of water through transpiration through the stomatal closure of plants, which is related to a stimulating effect of plant growth, due to the antiperspirant effect of chitosan 25). In turn, results found in bean cultivation have indicated that one of the aspects through which chitosan influences the reduction of transpiration is by increasing the levels of abscisic acid (ABA) in the leaves treated, which activates the partial closure of the stomata 26.
In this work, postures 25 days after germination did not reach the optimal size required for transplantation from15-18 cm 27. However, these results are similar to investigations carried out using the concentration of 1 g L-1 and imbibition for 4 hours, since the postures 24 days after germination differ from the control, but their heights do not exceed 9 cm 2. In the same way, it coincides with other works that when evaluating the effect of this product at this same concentration (1 g L-1) and by imbibition of tomato seeds of the cultivar "Mara" in the open air on stonemasons, achieve increases above the control from the 10 days of germinated the plants. However, it differs from the height reported by these researchers when they report that the positions are ready for transplanting 15 days after the plants have germinated 1. This work also differs from others that employ imbibition times of four and eight hours at different concentrations of chitosan (0.1-2000 mg L-1). In these investigations, the postures at 27 days of sowing did not show significant changes in the height of the plants in any of the concentrations evaluated with respect to the control, but in the dry mass of the root at the lowest concentration of 0.1 mg L -1 (28.
Taking into account that these are the first results of the chemical stability of QuitoMax® over time and its biological activity, it is suggested to extend the chemical stability until the year of conservation and to evaluate other morphoagronomic variables that may provide information on biological activity of the QuitoMax® preserved in tomato or other crops of economic interest.
The use of biostimulants in agriculture as opposed to the indiscriminate use of chemical fertilizers and pesticides is of vital importance because they contribute to decrease the polluting load of soils at the same time that they increase the yield and development of crops. QuitoMax® and chitosan derivatives are an efficient and promising route that must be taken into account in pursuit of the development of agriculture in our country.
CONCLUSIONS
The chemical analyzes carried out on the three batches of the QuitoMax® product did not demonstrate changes in the conductivity, pH and mass of soluble chitosan of the product during storage.
The QuitoMax® product shows biological activity after 270 days. The preserved product promotes advances in germination and increases in the height of tomato plants.
Taking into account the results obtained in this work, the use of the QuitoMax® can be extended up to 270 days.
BIBLIOGRAFÍA
1. Terry Alfonso E, Falcón Rodríguez A, Ruiz Padrón J, Carrillo Sosa Y, Morales Morales H. Respuesta agronómica del cultivo de tomate al bioproducto QuitoMax(r). Cultivos Tropicales. 2017;38(1):147-54. [ Links ]
2. González Peña D, Costales D, Falcón AB. Influencia de un polímero de quitosana en el crecimiento y la actividad de enzimas defensivas en tomate Solanum lycopersicum L. Cultivos Tropicales. 2014;35(1):35-42. [ Links ]
3. Costales D, Nápoles MC, Falcón AB, González Anta G, Ferreira A, Rossi A. Influencia de quitosanas en la nodulación y el crecimiento vegetativo de soya Glycine max L. Merrill. Cultivos Tropicales. 2017;38(1):138-46. [ Links ]
4. Rodríguez AF, Costales D, Peña DG, Morales D, Mederos Y, Jerez E, et al. Chitosans of different molecular weight enhance potato Solanum tuberosum L. yield in a field trial. Spanish journal of agricultural research. 2017;15(1):25. [ Links ]
5. Jerez Mompie E, Martín Martín R, Morales Guevara D, Reynaldo Escobar I. Efecto de oligosacarinas en el comportamiento de la papa Solanum tuberosum L. variedad Romano. Cultivos Tropicales. 2017;38(1):75-80. [ Links ]
6. Sotelo-Boyás ME, Valverde-Aguilar G, Plascencia-Jatomea M, Correa-Pacheco ZN, Jiménez-Aparicio A, Solorza-Feria J, et al. Caracterización de nanopartículas de quitosano adicionadas con aceites escenciales: Efecto in vitro en Pectobacterium carotovorum. Revista mexicana de ingeniería química. 2015;14(3):589-99. [ Links ]
7. Parvin N, Kader MA, Huque R, Molla ME, Khan MA. Extension of shelf-life of tomato using irradiated chitosan and its physical and biochemical characteristics. International Letters of Natural Sciences. 2018;67:16-23. [ Links ]
8. Nisha V, Monisha C, Ragunathan R, Johney J. Use of Chitosan as Edible Coating on Fruits and in Micro biological Activity-An Ecofriendly Approach. 2016;5(8):7-14. [ Links ]
9. Sucharitha KV, Beulah AM, Ravikiran K. Effect of chitosan coating on storage stability of tomatoes Lycopersicon esculentum Mill. International Food Research Journal. 2018;25(1):93-9. [ Links ]
10. Silva AM, Stamford TC, Souza P, Berger LRR, Leite MV, Nascimento AE, et al. AntifungalActivityofMicrobiologicalChitosanandCoatingTreatmentonCherryTomato Solanum lycopersicum var. cerasiforme toPost-HarvestProtection. 2015;4(9):228-40. [ Links ]
11. González-Peña Fundora D, Costales Menéndez D, Falcón Rodríguez A. Evaluación de indicadores que caracterizan la acción protectora del quitosano en Nicotiana tabacum L. vs Phytophthora nicotianae Breda de Haan. Cultivos Tropicales. 2015;36(3):144-53. [ Links ]
12. Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine drugs. 2015;13(3):1133-74. [ Links ]
13. Dimzon IKD, Knepper TP. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. International journal of biological macromolecules. 2015;72:939-45. [ Links ]
14. Al-Manhel AJ, Al-Hilphy ARS, Niamah AK. Extraction of chitosan, characterisation and its use for water purification. Journal of the Saudi Society of Agricultural Sciences. 2018;17(2):186-90. [ Links ]
15. Huerta AC, Rincón MC, Inciarte AC, López A. Obtención y caracterización de películas de quitosano elaborado a partir de los desechos de la industria cangrejera. Revista Iberoamericana de Polímeros. 2012;13(3):77-88. [ Links ]
16. Abramov AY, Kozyreva EV, Shipovskaya AB. Peculiarities of the physicochemical properties of chitosan solutions. European Journal of Natural History. 2013;(1):30-5. [ Links ]
17. Hernández JA, Pérez JJM, Bosch ID, Castro SN. Clasificación de los suelos de Cuba 2015. Mayabeque, Cuba: Ediciones INCA. 2015;93. [ Links ]
18. -León Moreno M. Evaluación de eficiencia de dos marcas diferentes de benzoato de sodio en zumo de naranja sobre pruebas microbiológicas [Internet]. Ricardo Palma, Perù; 2017 [cited 23/01/2020]. Available from: http://repositorio.urp.edu.pe/handle/urp/908 [ Links ]
19. Rinaudo M, Pavlov G, Desbrieres J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer. 1999;40(25):7029-32. [ Links ]
20. Bernabé-Galloway P, Argüelles-Monal W, Peniche-Covas C. Conductimetric study of the interpolyelectrolyte reaction between chitosan and pectin. Revista CENIC. Ciencias Químicas. 2012;43:1-7. [ Links ]
21. De Queiroz Antonino RSCM, Lia Fook BRP, De Oliveira Lima VA, De Farias Rached RÍ, Lima EPN, Da Silva Lima RJ, et al. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp Litopenaeus vannamei Boone. Marine Drugs. 2017;15(141):1-12. doi:10.3390/md15050141 [ Links ]
22. Zeng D, Luo X. Physiological effects of chitosan coating on wheat growth and activities of protective enzyme with drought tolerance. Open Journal of Soil Science. 2012;2(03):282-8. [ Links ]
23. Agbodjato NA, Noumavo PA, Adjanohoun A, Agbessi L, Baba-Moussa L. Synergistic effects of plant growth promoting rhizobacteria and chitosan on in vitro seeds germination, greenhouse growth, and nutrient uptake of maize Zea mays L. Biotechnology research international. 2016;2016:11. [ Links ]
24. Martínez González L, Reyes Guerrero Y, Falcón Rodríguez A, Núñez Vázquez M. Efecto del tratamiento a las semillas con quitosana en el crecimiento de plántulas de arroz Oryza sativa L. cultivar INCA LP-5 en medio salino. Cultivos Tropicales. 2015;36(1):143-50. [ Links ]
25. Lee Y-S, Kim Y-H, Kim S-B. Changes in the respiration, growth, and vitamin C content of soybean sprouts in response to chitosan of different molecular weights. HortScience. 2005;40(5):1333-5. [ Links ]
26. Iriti M, Picchi V, Rossoni M, Gomarasca S, Ludwig N, Gargano M, et al. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environmental and Experimental Botany. 2009;66(3):493-500. [ Links ]
27. Gómez O, Casanova A, Cardoza H, Piñeiro F, Hernández JC, Murguido C, et al. Guía Técnica para la producción del cultivo del tomate. Ministerio de la Agricultura, Instituto de Investigaciones Hortícolas" Liliana Dimitrova", Asociación Cubana de Técnicos Agrícolas y Forestales, Asociación Nacional de Agricultores Pequeños, Asociación Cubana de Producción Animal. Editora Agroecológica. La Habana, Cuba. 2010;18. [ Links ]
28. Martínez L, Castro I, Díaz L, Núñez M. Influencia del tratamiento a semillas con quitosana en el crecimiento de plantas de tomate Solanum lycopersicum L. Cultivos Tropicales. 2007;28(4):79-82. [ Links ]
Received: December 11, 2018; Accepted: February 18, 2020











 texto en
texto en 


