INTRODUCTION
The human parasitism by intestinal helminths and protozoa is still an important public health issue, particularly among people who live in conditions of poor housing and sanitation.1 Approximately 2 billion people are infected by soil-transmitted helminths, which more often are represented by Ascaris lumbricoides, hookworm and Trichuris trichiura.2 Among intestinal protozoa, the most important human infections are amoebiasis, cryptosporidiosis, and giardiasis.3
The occurrence of intestinal parasites is closely related to the environmental, social and sanitation conditions. Soil temperature and moisture are critical for the embryogenesis of helminths. Poverty, associated to lack of treated water and wastewater treatment, favors the maintenance of contamination in water and soil, which leads to successive reinfections of people in endemic areas.2 Cultivation of vegetables consumed raw in areas where soil and water contamination by feces occurs may represent a way to human infection by intestinal parasites or commensals.4
In Brazil, traditional communities composed of people descending from Afro-Brazilian former slaves sharing a territory and cultural traits, known as ‘quilombola’ communities,5 represent people that live in vulnerable conditions, with difficult access to health, education, treated water or garbage collection services.6,7,8,9,10 This situation contributes to the occurrence of several parasitic diseases, including the intestinal ones.6
Cirqueira-Júnior et al.8 studied the domiciliary structure and the occurrence of intestinal parasites reported by health professionals, in the ‘quilombola’ community of Quartel do Indaiá, located in the Minas Gerais State. These authors stated that houses have good living conditions; however, sanitation structures are precarious or even absent. Additionally, the same study reports the continuous occurrence of ascariasis among children and adults, with frequent findings of worms in feces, and oral and nasal cavities, suggesting high environmental contamination.
Considering this situation, the present study aimed to verify the occurrence of intestinal parasites among people, the environmental contamination by intestinal parasites in soil and vegetables, and fecal contamination of water in the ‘quilombola’ community of Quartel do Indaiá, Minas Gerais State, Brazil.
METHODS
Study area
This study was carried out at the community of Quartel do Indaiá (Latitude: -18.02; Longitude: -43.735; Elevation: 970 m.a.s.l.), located in the municipality of Diamantina, State of Minas Gerais, Brazil. This community is recognized as reminiscent of a quilombola-community.11 In 2011, 126 people, of 25 families, were living there. Most of the houses did not have bathrooms, and the wastewater was released directly in the soil or the rivers that surround the community.8 Economics is based on agriculture and cattle rearing, and the cultivation of kitchen garden is a common activity among the inhabitants.
There is a primary school in the community, and the nearest health facility is located in the São João da Chapada village, nine kilometers afar.
Parasitological analysis of feces, soil and vegetables
Occurrence of intestinal parasites and commensals was evaluated by means of coprological analysis. Fecal samples were collected in vials containing formaldehyde solution (10 %) and processed by spontaneous sedimentation, according to Hoffman et al.12 Briefly, feces (~5 g) were homogenized with water and filtered in gauze to a 250 mL sedimentation glass. The volume of the glass was filled with water and the solution remained in sedimentation for at least 6 h. A drop of final sediment was analyzed in triplicate under light microscope at 400x magnification (Olympus CX41, Tokyo, Japan). An iodine solution was used for better visualization of cysts.
Soil samples were collected from the ground’s surface (maximum 5cm depth) and stored in plastic bags. Three samples were collected in the surroundings of the houses (~2 m), three samples were collected in the surroundings the sewer (latrines or the places that the habitants used to defecate directly on soil), and three samples were collected in the kitchen garden, wherever there was presence of it. The samples comprised approximately 50 g of soil, and 20 g were processed by spontaneous sedimentation.13 The procedure was similar to the described for fecal samples. Sediment was analyzed in triplicate under light microscope at 400x magnification (Olympus CX41, Tokyo, Japan).
Vegetables were collected according to availability from the kitchen gardens of the community. They were donated by the owners and stored in plastic bags at 6°C until analysis, which occurred at a maximum 48 h interval after collection. Analyses were carried out according to Luz et al.14)
The vegetables leaves were separated and those damaged were discarded. Remaining leaves were individually washed with a detergent solution (0.5 % v/v) of Tween 80 in 0.85 % NaCl saline solution. Washing liquid was filtered through gauze in a sedimentation glass. After 24 h sediment was centrifuged (855 x g; 2 min). The final sediment was analyzed in triplicate under light microscope at 400x magnification (Olympus CX41, Tokyo, Japan). Vegetables were collected throughout a year, depending on availability and culture season.
Fecal samples were collected between April 2013 and June 2013, soil samples were collected between April 2013 and May 2013, and vegetable samples were collected between April 2013 and July 2014. For all sampled materials, the intestinal coccidian occurrence was not evaluated.
Microbiological analysis of water
Because water used by people of Quartel do Indaiá for consumption and irrigation is not treated, a microbiological analysis was proposed, to verify fecal contamination. Five water samples were collected in the community. Four 100 mL samples were obtained at the two main watercourses (the Caeté-Mirim and Luiz Carlos rivers) surrounding the community (one sample before the river enters the community and another one immediately after the river leaves the community), and one sample was collected from the water tank that supplies most of the houses in the community. Samples were collected in sterile culture bottles and added with ColiTag® chromogenic substrate. They were incubated at 35 °C (± 0.5 °C) for 24 h and, afterwards, analyzed according to the manufacturer instructions. This method presents good performance in the evaluation of water collected from rivers and from public supply.15 A sample with sterile water was used as negative control. Positivity for total coliforms was confirmed by yellow coloration, and positivity for Escherichia coli was confirmed when samples became fluorescent at UV light (365 nm) exposition. Negative control remained colorless. The water samples were collected in August 2014.
Ethical statements
This study followed the Brazilian ethics statements, and was approved by the Ethics Committee of Universidade Federal dos Vales do Jequitinhonha e Mucuri, under the protocol number 078/2012. Informed consent was obtained from the participants or from parents or legal guardians, in the case of participants under legal age. All people with positive diagnosis for intestinal parasites received free medical consultation and treatment.
RESULTS
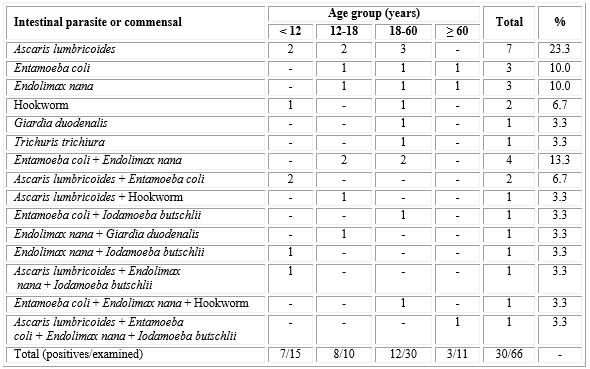
During the collection of feces, 78 people able to participate were present in the community, and 66 agreed and provided samples. The participants included 32 males and 34 females, with ages ranging from less than one year to 86 years old. Occurrence of intestinal parasites and/or commensals was observed in 30 (45.5 %) samples, affecting people of all age groups. Main parasites and commensals were A. lumbricoides, Entamoeba coli, and Endolimax nana, which appeared in 12 (18.2 %) samples each. In 13 (19.7 %) samples, more than one parasite or commensal were observed (table 1).
Table 1 Intestinal parasites and/or commensals according to age group among people of the quilombola community of Quartel do Indaiá, Minas Gerais State, Brazil, 2013
Among the 25 houses existing in the community, soil samples were collected in 19. The remaining houses were uninhabited, or the residents did not provide feces samples, so they were excluded of soil and vegetables collection in this study. The soil analysis revealed contamination in 26 samples (20.6 %), from a total of 126 (13 positives/57 analyzed around the houses, 8/45 in the waste, 5/24 in kitchen gardens) from 39 sites. The positive sites and the contaminants are shown in table 2, which presents the contamination by cysts of E. coli, E. nana and I. butschlii, and eggs of A. lumbricoides and Enterobius vermicularis.
Table 2 Intestinal parasites or commensals detected in the soil of the quilombola community of Quartel do Indaiá, Minas Gerais, Brazil, according to positive collection site, 2013

Vegetables were obtained from seven kitchen gardens. Contamination was observed in 75 out of 135 samples among seven of eight analyzed vegetables (table 3). The contaminants were eggs of A. lumbricoides and hookworm, and cists of E. coli.
Table 3 - Contamination of vegetables by intestinal parasites or commensals in the quilombola community of Quartel do Indaiá, State of Minas Gerais, Brazil, according to vegetable and contaminant, 2013-2014

The distribution of the occurrence of intestinal parasites or commensals among people, in soil and vegetables in the community of Quartel do Indaiá was spread over the community, as shown in the figure.
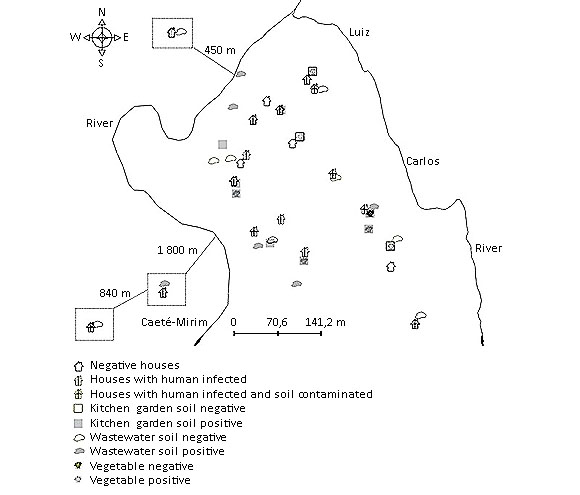
Fig Distribution of occurrence of intestinal parasites and/or commensals in human, soil and vegetables in the ‘quilombola’ community of Quartel do Indaiá, State of Minas Gerais, Brazil.
The microbiological assessment of water revealed that all samples were contaminated by total and fecal coliforms.
DISCUSSION
The Brazilian rural communities face difficulties to access treated water and sanitary structures, a fact that may favor the contamination and infection by intestinal pathogens and commensals. The traditional communities, such as the ‘quilombola’ and indigenous communities, have been historically marginalized, and present high occurrence of diseases related to lack of sanitation.6
In the ‘quilombola’ community of Quartel do Indaiá, the positivity for intestinal parasites or commensals reached almost half of all examined people. These results are in accordance with previous observations among other ‘quilombola’ communities in the States of Minas Gerais,16 Bahia,6 and Espírito Santo,17 where positivity was observed among 63.8 %, 35.4 %, and 42.7 % of all examined people, respectively.
The positivity for intestinal parasites/commensals was detected in all age groups, corroborating the observations of Cirqueira-Júnior et al.8 for analysis of medical records from the same community. Intestinal parasitism tends to decrease with age,18 but in places that maintain high environmental contamination, this situation may not occur.19 It is also remarkable that 43 % of positive people presented more than one intestinal parasite/commensal, including a person aging more than 60 years old who presented four different species.
A. lumbricoides was the most frequently detected intestinal parasite, occurring in all age groups. It represents the main intestinal helminth infecting people around the world,2 and its presence is closely related to inadequate sanitary conditions.20 Among protozoa, the commensals, E. coli and E. nana, were the most frequently detected. Although they do not represent immediate risk to human health, the detection of intestinal commensals indicates the intake of cysts, as they share the same route of the parasitic species.21
Analyses of vegetables, soil and water confirmed the high environmental contamination by fecal material in the community of Quartel do Indaiá. This situation probably results from the lack of sanitation observed in this community, in which all households release sewerage directly at the soil or the rivers,8 as also observed among indigenous communities of the Paraná State, Brazil, where peridomiciliar soil contamination was directly associated to the absence of bathrooms and the presence of feces on the soil.22,23 The finding of contamination of soil only in the surroundings of houses with infected people in Quartel do Indaiá reinforces this idea.
The contamination of food and water associated to poor hygiene habits may favor reinfection by intestinal parasites. Many studies have demonstrated that vegetables may carry protozoan cysts or helminth eggs.24,25,26 Since the investigated vegetables are often for used for raw consumption, if their sanitization is precarious, they may possibly serve as infection sources for intestinal parasites.27
The results presented high environmental contamination in Quartel do Indaiá. However, it is probable that the contamination is even higher, as the collection of soil and water, for example, was done only once for each site. The human infections may as well be underestimated, as the diagnosis was based on only one method and using a single sample.28 Nevertheless, these findings may contribute to better understanding of the health and sanitary conditions among traditional communities in Brazil, supporting health policies to solve those issues.














