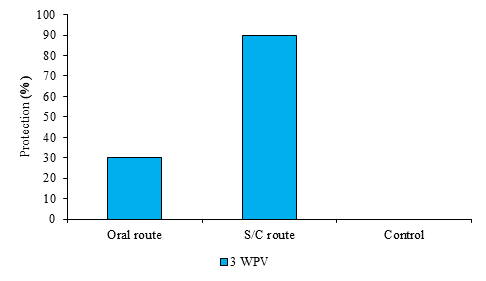Introduction
Rabbit industry is very important because its meat is considered a delicacy because of its high palatability and nutritive value. However, it currently faces several constraints threatening its growth and continual development. The emergence of bacterial and viral pathogens causes huge losses. Rabbit hemorrhagic disease virus (RHDV) and Clostridium perfringens type A have gained particular concern in this field.
Rabbit hemorrhagic disease (RHD) is a highly fatal viral disease that causes heavy losses among rabbits. The newly emerging RHDV2 was detected in vaccinated flocks in mid-2018.1 RHDV2 infected rabbits developed sudden death and blood stains on the nose caused by internal bleeding with high morbidity and an average mortality ranged from 5-70%. After that, the pathogenicity of RHDV2 increased with an adult mortality rate ranging from 70 to 100%.2 Rabbits infected by ancient isolated RHDVs, either classical or variants, show high morbidity and mortality rates in adult rabbits, but young rabbits less than 6-8 weeks old are less susceptible; RHDV2 is able to infect young rabbits (15-25 days of age).3 Enterotoxemia or bloat is a severe diarrheal disease that primarily affects 4 to 8 week old rabbits that are naturally infected and it can also affect rabbits of any age with mortality rates of up to 50%; it is characterized by lethargy, rough coat, greenish brown fecal material covering the perineal area, and death within 48 hours and the primary causative agent is C. perfringens type A.4
Vaccination of rabbits is widely used to control bacterial and viral infections. Using autogenously inactivated culture against RHDV and toxoid vaccine against clostridial infection has been extensively used over the past decades. Using combined vaccines has the advantage of protecting against more than one disease at the same time, enhancing the immune response of the vaccinated rabbits, reducing vaccination expenses and decreasing the stress of vaccinating with different vaccines.5 Inactivated vaccines adjuvanted by aluminum hydroxide gel or Montanide ISA 70 VG can provide satisfactory protection against these infections.6,7 The use of Montanide™ ISA series as adjuvant has increased after being tested in different vaccine formulations for different animal species and proving to be safe and efficacious. For example, IMS 1313 N VG PR is an aqueous-based nanoparticles adjuvant that has generated superior immunostimulant activity against veterinary vaccines. It is also eligible for mass oral delivery and parental vaccination, which can enhance immune protection in different animal species.8 Lysozyme and nitric oxide (NO) are important innate immune parameters. Lysozyme plays several roles in host defense mechanisms including antibacterial, antiviral, and immunomodulatory effects.9 In addition, NO, a free radical that acts as a pro-inflammatory cytotoxic mediator and is produced by inducible nitric oxide synthases (iNOS) in activated macrophages and neutrophils, regulates a number of immunological and physiological processes.10 It is generally known that in poultry and mammals, the activation of the innate immune system is characterized by the production of inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-ɑ (TNF) that are an integral part of the immune response to vaccination.11 Therefore, the present study was designed to prepare and asses the efficacy of a combined inactivated vaccine against RHDV2 and C. perfringens type A infections, adjuvanted with Montanide™ ISA 1313, using different administration routes (subcutaneously and oral) in susceptible rabbits.
Materials and Methods
Ethical approval
Animal subject
All methods were performed according to relevant guidelines and regulations. All experiments were carried out according to ARRIVE 2.0 guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) in the Faculty of Veterinary Medicine, Cairo University (Code: VetCU01102020217).
Experimental animals
Rabbits
One hundred and fifty-four, seronegative New Zealand White rabbits, males, as 45 days old were purchased from a conventional rabbit try in Qalubia governorate. Rabbits were required for vaccine evaluation.
Mice
Two hundred Swiss white mice (18-20 gm) were obtained from CLEVB. They were used to evaluate the potency of C. perfringens type A in the prepared vaccines.
Strains
Standard C. perfringens type A antitoxin
C. perfringens type A antitoxin was obtained from National Institute for a Biological Standard Control, United Kingdom. It contains 270 IU/mL alpha antitoxin.
Inactivation of virus and bacteria
RHDV2 suspension was inactivated using formalin with a final concentration of 2% of the total volume for 48 h.6
Culture suspension from highly toxigenic isolates of C. perfringens type A was inactivated by formalin in 0.5% concentration.7
Assessment of virus and bacteria inactivation was achieved through injection of each inactivated suspension into five rabbits and keeping two rabbits as controls. If the inoculated rabbits did not subsequently show clinical signs of diseases like RHDV2 (sudden death and blood stains on the nose) and enterotoxemia (lethargy, rough coat, greenish brown fecal material covering the perineal area) or mortality, each inactivated suspension was considered ready to be emulsified with the vaccine adjuvant.7
Adjuvant
It was used Montanide™ IMS 1313 N VG PR (IMS 1313; SEPPIC, France) to formulate a combined vaccine; it was mixed with an equal volume of inactivated suspension of RHDV2 and C. perfringens type A strain cultures with a ratio of 50:50 as recommended by the manufacturer.
Vaccine preparation7
Two equal inactivated suspensions (to occupy 50% of preparation volume) were adjuvanted by Montanide™ IMS 1313 N VG PR (to occupy 50% of remaining preparation volume). The recommended vaccine dose was adjusted to contain 210 HAU from inactivated suspension of RHDV2 and 60 Minimum Lethal Dose (MLD) of C. perfringens alpha toxoid per vaccine dose. The vaccine was administrated orally and subcutaneously (S/C) with a dose (0.5 mL/animal).
Erythrocyte suspension
Erythrocytes human type "O" were collected from a healthy volunteer (with prior informed consent of the volunteer). A 3.8% sodium citrate solution was use as anticoagulant. The packed erythrocytes were suspended in sterile saline in a concentration of 0.75% for micro-technique of HA and Hemagglutination inhibition (HI) tests.3
Evaluation of combined prepared vaccine
Sterility test
It was done to determine if the vaccine samples were free of bacterial, fungal and mycoplasma contaminants and acceptable for release. All vaccine vials were tested individually. One mL of each vaccine sample was inoculated into bacterial and fungal media plates. The inoculated media were incubated aerobically and anaerobically for 21 days at different degrees of temperature. Inoculated media were inspected for possible growth.3,4
Safety test
Ten seronegative rabbits were inoculated with double dose of prepared candidate inactivated vaccine subcutaneously and orally (five rabbits each route). Two control rabbits were kept along the test. Vaccinated rabbits were observed for any possible local or systemic adverse reaction due to vaccine for 21 days.
Potency test
Evaluation of the immune response to C. perfringens type A
It was done using Toxin neutralization test.7 The mean C. perfringens type A alpha antitoxin titers in sera from vaccinated rabbits were measured using Toxin neutralization test (IU/mL) which was applied in mice.
Evaluation of the immune response to RHDV2
The HA test was done to determine the HAU for RHDV2 antigen.3
The HI test3 was done to estimate specific RHDV antibodies in rabbit sera. The test was carried out using 8 HAU of RHDV and human RBCs type "O".
Ten rabbits from each group were challenged (3 intramuscularly at a dose of 1 mL/rabbit with 100 LD50 of local RHDV2 after 3rd weeks post vaccination (WPV). Observations for clinical signs and death were carried out for 14 days post challenge.
Protection (%) = Number of survivals/Total number of challenged rabbits X 100.
Measuring of serum nitric oxide (NO)13
Serum proteins were precipitated by centrifuging 100 µL of the serum sample at 13,000 rpm for 20 min after mixing it with 80 µL of ZnSO4 and 120 µL of NaOH. Supernatant was obtained and added to 400 mg of copper-plated cadmium, adding 100 µL of 0.2 M glycine buffer and shook for 2.5 h. Griess reagent (100 µL) was added to 100 µL of supernatant in a 96-well ELISA plate. The optical density was determined at 545 nm with an ELISA plate reader. NO concentration was calculated from the standard curve of NaNO2 solution.
Determination of IL-6 and TNF-α mRNA in spleen tissue by quantitative real time-PCR (qRT-PCR)
RNA extraction and purification from spleen samples were performed using QIAamp RNeasy Mini kit (Qiagen, Germany, GmbH). The used oligonucleotide primers of IL-6 and TNF-α were illustrated in Table 1 (Metabion, Germany). The SYBR green qRT-PCR reaction was performed in a Stratagene MX3005P real time PCR machine and the analysis of qRT-PCR results were determined by using the amplification curves and threshold cycles (CT) values (stratagene MX3005P software). To estimate the differences of fold changes in the mRNA gene expression of the different samples, the CT of each sample was compared with that of the positive control group according to the "-2ΔΔCt” method.14
Table 1 Primer sequences for quantitative real time-PCR.
| Target gene | Primers sequences | References |
|---|---|---|
| GAPDH* | TGACGACATCAAGAAGGTGGTG | Schnupf P, et al.15 |
| GAAGGTGGAGGAGTGGGTGTC | ||
| IL-6 | CTACCGCTTTCCCCACTTCAG | |
| TCCTCAGCTCCTTGATGGTCTC | ||
| TNF-α | GTCTTCCTCTCTCACGCACC | Godornes C, et al.16 |
| TGGGCTAGAGGCTTGTCACT |
*GAPDH: glyceraldehyde 3-phosphate dehydrogenase, served as an internal control for sample normalization.
Experimental design
One hundred and fifty-four, seronegative rabbits divided into three groups (group 1, group 2 and group 3) were used in this study. Groups 1 and 2 were composed of 12 rabbits (each) used for assessing complete inactivation of virus and bacteria and testing vaccine safety, respectively. Group 3 was composed of 130 rabbits used for evaluation of the prepared vaccine which was divided into 3 subgroups as follows:
Subgroup 1 (50 rabbits): vaccinated with combined vaccine S/C.
Subgroup 2 (50 rabbits): vaccinated with combined vaccine oral one drop.
Subgroup 3 (30 rabbits): kept as non-vaccinated challenged group (control +ve).
Ten and five rabbits from each of the vaccinated and control groups, respectively, were bled weekly till 4thWPV. Sera collected from vaccinated and control rabbits were tested for detection of RHDV2 HI antibodies using HI test; the Toxin neutralization test was applied in mice for determination of antitoxin titer for C. perfringens type A. Calculation of lysozyme activity and NO were achieved through examination of collected antisera at 1st, 2nd, 3rd and 4th WPV. Spleen tissues of vaccinated rabbits were examined at 1st and 2nd WPV using quantitative real time-PCR (qRT-PCR) for determination of IL-6 and TNF-α mRNA. Challenge test against RHDV2 infection was performed at 3th WPV in ten and five rabbits from each vaccinated group and control, respectively.
Results
Assessment on virus and bacteria inactivation
All rabbits injected with formalin-treated virus and bacteria were kept alive without any clinical signs.
Sterility and safety
The tested vaccine was free from bacterial and fungal contamination. Furthermore, the prepared vaccine was found safe and no clinical symptoms appeared in seronegative rabbits, after S/C and oral inoculation with double recommended dose.
Potency
Evaluation of the immune response to C. perfringens type A
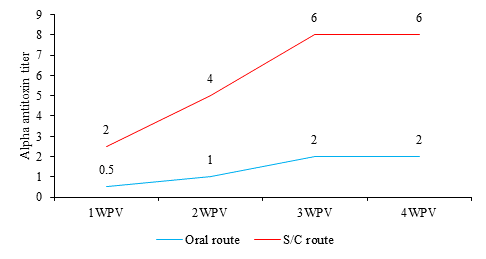
The mean C. perfringens type A alpha antitoxin titers in sera from vaccinated rabbits (IU/mL) are shown in Figure 1. Mean C. perfringens type A alpha antitoxin titer in sera from rabbits vaccinated orally was 0.5 IU/mL at 1st WPV and reached to 2 IU/mL at 3rd WPV. While the antitoxin titer of vaccinated rabbits by S/C was 2 IU/mL at 1st WPV and gradually increased until reach 6 IU/mL at 4th WPV.
Evaluation of the immune response to RHDV2
Hemagglutination inhibition test for RHDV2
As shown in Figure 2, mean RHDV2-HI antibody titers were 2, 3, 3.5 and 3.5 in sera from orally vaccinated rabbits at 1st, 2nd, 3rd and 4th WPV, respectively; while rabbits that were vaccinated by S/C route had higher immune response, with 5, 7.3, 8 and 8 HI titers at 1st, 2nd, 3rd and 4th WPV, respectively.
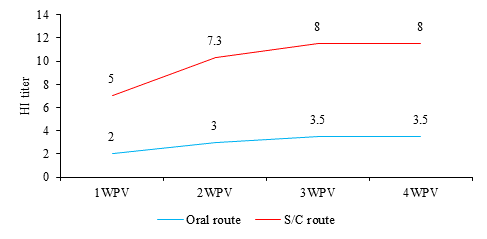
Fig. 2 Mean RHDV2-HI antibody titers in sera of vaccinated rabbits at 1st, 2nd, 3rd and 4th weeks post vaccination (WPV). S/C route: subcutaneous route.
Protection percent against RHDV2 challenge
The percentage of protection for the orally vaccinated rabbits challenged at 3rd WPV recorded 30%, while the S/C inoculated group had a higher protection percentage, reaching 90% at 3rd WPV (Figure 3). All control rabbits died with RHDV2-specific lesions after 48 h.
Lysozyme activity
The results of serum lysozyme assay in rabbits (Table 2) showed marked increase in both oral and S/C route compared to the control group. Administration by the S/C route induced the highest level of lysozyme at all time intervals, the increase was significant compared to the control at all time intervals; it also induced a significant increase compared to oral route at 2nd and 3rd WPV. While the oral route induced a significant increase compared to control group only at 1st, 2nd and 3rd WPV.
Table 2 Serum lysozyme test results.
| Serum lysozyme level (µg/mL) | |||
|---|---|---|---|
| Week post vaccination (WPV) | Control | Oral route | S/C route |
| 1st WPV | 80.25 ± 4.95a | 122.35±13.99b | 139.28±8.75b |
| 2nd WPV | 83.75±5.45a | 131.28±8.75b | 152.03±2.47c |
| 3rd WPV | 81.25±5.90a | 144.30±3.70b | 196.35±24.65c |
| 4th WPV | 79.60±7.80a | 90.05±5.05ab | 109.95±4.95b |
Data are presented as the mean ± SE. Means with different superscript small letter indicate significantly different at the same row between groups at P < 0.05 using one-way ANOVA test.
Serum nitric oxide levels
Regarding the results of NO assay (Table 3), both vaccination routes induced higher NO values than the control group. The S/C route induced the highest significant increases in values for all groups at all time intervals. However, the oral route induced significant increased values compared to the control group only at 1st and 2nd WPV.
Table 3 Serum nitric oxide assay results.
| Serum nitric oxide level (µmol/mL) | |||
|---|---|---|---|
| Week post vaccination (WPV) | Control | Oral route | S/C route |
| 1st WPV | 11.6 ± 0.27a | 16.1±1.28b | 25.59±1.15c |
| 2nd WPV | 10.71±0.36a | 17.37±2.15b | 25.90±3.91c |
| 3rd WPV | 11.23±2.17a | 13.75±2.79a | 21.1±1.15b |
| 4th WPV | 10.90±0.12a | 13.20±1.03a | 15.27±1.14b |
Data are presented as the mean ± SE. Means with different superscript small letter indicate significantly different at the same row between groups at P < 0.05 using one-way ANOVA test.
Cytokine mRNA gene expression of IL-6 and TNF-ɑ in rabbit spleen
The results of mRNA of IL-6 and TNF-α in rabbits’ spleen are illustrated in Table 4. The S/C route induced the highest values at all time intervals. There were significant increased values of IL-6 at 2nd WPV and significant higher values of TNF-ɑ in S/C group at 1st and 2nd WPV compared to the oral route group.
Table 4 Results of cytokine mRNA gene expression of IL-6 and TNF- ɑ.
| Cytokines mRNA gene (fold change) | |||
|---|---|---|---|
| Cytokines | WPV | Oral route | S/C route |
| IL-6 | 1st WPV | 6.68±0.35 | 7.01±0.53 |
| 2nd WPV | 8.28±0.69a | 13.55±0.43b | |
| TNF-ɑ | 3rd WPV | 4.66±0.51a | 6.68±0.5b |
| 4th WPV | 6.49±0.35a | 11.79±0.17b |
Quantitative real-time-PCR for mRNA expression of IL-6 and TNF-ɑ genes in rabbits. 2−ΔΔCt method was used to calculate the relative expression fold change. Data are presented as the mean ± SE. Means with different superscript small letter indicate significantly different at the same row between groups at P < 0.05 using one-way ANOVA test.
Discussion
Vaccination has become an effective and commonly used strategy to prevent spread of disease among rabbits.6,7 The use of bivalent vaccines provides better protection compared to monovalent vaccines, besides making vaccination more economical and cost-effective.5) RHDV2 and C. perfringens type A are among the main responsible for morbidity and mortalily, especially among young rabbits.1,4 This study aimed to develop and evaluate a combined inactivated vaccine against RHDV2 and enterotoxemia adjuvanted with Montanide™ IMS 1313 using different routes of vaccine administration (subcutaneous and oral). Using nanoparticle adjuvants as (Montanide™ IMS) is increased nowadays due to contain an immunostimulating compound and their potential ability to enhance both humoral and cell-mediated immunity.17 The using of Montanide as an adjuvant in this study enhances the advantages of the vaccine and can facilitate antigen uptake by Antigen Presenting Cells, which presumably improves antigen processing and presentation via major histocompatibility complex (MHC) class II and class I. The use of this type of adjuvant to protect young rabbits from infection, due to its ability to exhibit early and satisfactory immune response is consistent with a study8 that demonstrated the ability of Montanide™ Seppic IMS1313 to enhance immune protection in different animal species. Furthermore, oral administration of the prepared vaccine in the present study is supported by a study of other authors,18 who said that the Montanide™ Seppic IMS1313 adjuvanted vaccine may also be eligible for oral mass vaccination.
Evaluation of the prepared combined vaccine was done according international protocols as OIE, which include assessing sterility, safety and potency. The prepared vaccine was free of any contamination without any adverse effect on vaccinated rabbits. Concerning vaccine potency, individual blood samples were collected weekly from all rabbit groups and examined against RHDV2 and C. perfringens type A alpha antitoxin titers using HI and Toxin neutralization tests, respectively. Antibody titers against RHDV2 antigen exhibited in orally vaccinated rabbits were very low and did not reach a satisfactory antibody level according to OIE,3 which requires ≥6 log HI antibodies in serum samples collected 3-4 weeks after vaccination for approval. Also, the mean C. perfringens type A alpha antitoxin titers in sera from the same vaccinated rabbits measured by Toxin neutralization test did not reach the protective titer until the 4th WPV according to United States Department of Agriculture (USDA),19 which determines that the satisfactory titer is not lower than the permissible limit (4 IU/mL) to be valid. Previous data revealed the oral administration of the vaccine can stimulate the immune system, but without an efficient role in producing a satisfactory level of antibodies against RHDV2 or C. perfringens type A, these results were consistent with others8 that proved the oral route of delivery had practical limitations, including low pH, gastric proteolytic enzymes, rapid transit through the intestine and poor absorption of large molecules. While in subcutaneously inoculated rabbits there was a gradual increase in antibody titer, with a satisfactory immune response being achieved from the 2nd WPV, these results agreed with those of other authors,20 who found that Montanide oil IMS 1313 VG NP-based vaccine induced a protective neutralizing serum antibody titer from the 2nd WPV, reached the highest level at the 3rd month post vaccination (MPV) and persisted at protective level for 9th MPV. After 21 days post vaccination, ten rabbits from each group were challenged intramuscularly at a dose of 1 mL/rabbit containing 100 LD50 of the homologous RHDV2; it was found that S/C vaccinated rabbits provided satisfactory protection recording 90%, while orally vaccinated rabbits had low protection 30%; these data agreed with another study,21 which found that IMS 1313 N adjuvanted vaccines conferred improved protection to vaccinated animals compared to the commercial reference.
In the current study, the levels of lysozyme and NO were increased along the experimental intervals in the vaccinated groups compared to the control group. These results agree with findings of other authors,22) who found an increase in the lysozyme level in rabbits at 14th and 30th day after S/C RHV vaccination and increased phagocytic capacity of polymorphonuclear cells, which may increase their function and, therefore, the production of NO from these cells. Moreover, vaccination against C. perfringens type C, D and C. oedematiens type B and C3 genotypes in dairy sheep influenced humoral factors of innate immunity and increased the lysozyme level.
IL-6 and TNF levels increased parallel to the increases in the innate immune parameters (lysozyme and NO) tested for the oral and S/C routes compared to the control; significant highest values were recorded for the S/C route. These increased levels of pro-inflammatory cytokines agreed with other study23) where S/C vaccination with the viral hemorrhagic septicemia vaccine increased the level of cellular immune response and the level of cytokines (IL-6 and TNF-α) in the rabbit thymus.
Conclusions
The prepared vaccine was safe, sterile and free from any contaminant. Vaccination by S/C route induced high HI antibody and antitoxin titers against RHDV2 and C. perfringens type A, respectively, in addition to a high percentage of protection against RHDV2 challenge compared to the oral route. Lysozyme, NO, IL-6 and TNF-α in rabbits vaccinated subcutaneously were optimum compared to the oral route.