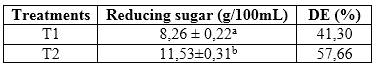Introduction
Malt syrup is used to prepare candies, jams, beverages, beer, it is also used in the pharmaceutics industry.1 The main source for obtaining malt syrup is barley, in which starch is transformed into malt during germination, but this cereal does not grow in all climates and many countries need to import it.2 A suitable and commercial source of starch is Cassava. Cassava, Manihot esculenta crantz is a tuberous plant which grows best in tropical and subtropical areas of the world all through the year.
There is an artisanal process that use cassava starch and amylase enzymes from rice seedlings for the production of maltose syrup. However, amylase from other vegetables could be used in the process.
Many alternatives have been sought to replace barley, achieving malt from rice, wheat, maize and sorghum by germination and subsequent treatment at different temperatures.3 These variants have a disadvantage, cereals are an important part of human and animal feed, which limits their use for this purpose. Another important source of starch is tubers, from which malt and glucose syrups can be obtained by acid or enzymatic hydrolysis. To perform enzymatic hydrolysis, more efficient and harmless, it is necessary to use pure amylase or mix germinated cereals as sources of amylases, with the starch obtained from the tubers.
Sweet potato (Ipomea batatas) has a high starch content, but the presence of latex and fiber limits its extraction, however, it has a considerable amount of β-amylase, one of the enzymes that hydrolyzes starch. Sweet potato is reported to contain high level of amylase activity (146 UmL-1) among the plants.4
β-amylase hydrolyzes soluble starch or amylose, yielding maltose as an end product. Hydrolysis of soluble starch or amylose by β-amylase occurs randomly, releasing a large number of oligosaccharides or dextrin. The dextrin is then hydrolyzed, yielding a large quantity of maltose and little glucose and maltotriose.5
The native amylases have important and well-documented influences in sweet potato storage and processing.6 It is known that in baked sweet potato roots, endogenous amylases hydrolyse part of the starch into maltose and longer chain polysaccharides, resulting in a sweet taste.7 The enzymes of sweet potatoes (β-amylase) are efficient and more stable to heat than that of barley.8) The use of β-amylase extracted from sweet potato in the determination of starch in food has been reported, but not in the preparation of malt syrup from cassava starch.9
Maltose syrup is produced by hydrolysis of starch. In the past, hydrolysis of starch was widely carried out using acid. However, acid hydrolysis are generally energy intensive, relatively difficult to control, require the use of materials that do not corrode easily, give rise to high colour and salt-ash content (after neutralization).
This paper describes the use of cassava (Manihot esculenta), as a source of starch, due to its high content of polysaccharide and, in addition, tubers declared unfit for consumption can be used. As a source of β-amylase, sweet potato was used, a tuber that has a high content of the enzyme, which can be extracted by simple methods. The hydrolysis of starch was developed under laboratory conditions, obtaining maltose syrup with amounts of reducing sugars within the established parameters.
Materials and methods
Preparation of starch
1 kg of cassava (CENSA 74-6329 variety) was used, from which the bark was previously removed, then crushed in a laboratory mill (IKA, Germany), and pressed for 24 h. The filtrate was allowed to settle overnight, and the supernatant liquid was decanted. The resulting precipitate was dried in an oven (WSU 400, Germany) at 60 °C for 24 h.10
Preparation of enzymatic extract
100 g of sweet potatoes were then homogenized in a blender for three minutes with 300 mL of cold extraction buffer consisting of 20 mM sodium phosphate (pH 6,0), and containing 0,3 % sodium chloride, 0,2 % calcium chloride, then filtered through four layers of cheesecloth. This extract was centrifuged at 13,000 x g for 10 min, and the supernatant removed and kept on ice for further use.9
Purification by Ammonium sulphate precipitation
Enzyme solution was mixed with a buffer phosphate 50 mM and the pH was adjusted to 6. Then 50 mL of the mixture were saturated by ammonium sulphate from 30 % w/v to 80 % w/v. The salt was mixed thoroughly and then the salt enriched solution was incubated at 4 °C for 8h. The saturated solution was centrifuged at 10 000 x g for 20 min. Supernatants were discarded, and the precipitates were dissolved in the phosphate buffer at pH of 6,5.11
Quantification of proteins in β-amylase preparations
Protein concentration was determined by Bradford method, using bovine serum albumin (BSA) as standard.12
Protein solution containing 2 to 5mg of bovine serum albumin (BSA) in a volume up to 1 mL was pipetted into 7 test tubes. The pH was adjusted to 5,5 with a buffer phosphate. 5 mL of protein reagent were added to the test tube and the contents mixed by vortexing. The absorbance was measured at 280 nm after 2 min and before 1 h against a reagent blank prepared from 5 mL of the appropriate buffer and 5 mL of protein reagent. The mass of protein was plotted against the corresponding absorbance resulting in a standard curve used to determine the protein in unknown samples.13
Preparation of protein reagent: Coomassie Brilliant Blue G-250 (100 mg) was dissolved in 50 mL 95 % ethanol. To this solution 100 mL 85 % (w/v) phosphoric acid was added. The resulting solution was diluted to a final volume of 1 L.13
Hydrolysis of Starch by using β-amylase from sweet potatoes
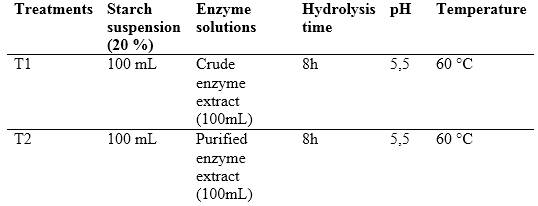
It was started from 100 mL of a suspension of cassava starch at 20 % w/v, to which the pH was adjusted to 6 by adding Ca(OH)2 0,1 N. Subsequently, 50 mL of the enzyme extract was added, and the mixture was gradually heated in a water bath under stirring at 60 °C. After 2 h another 50 mL of the enzyme extract were added, the mixture was kept at 60 °C for 6 h under stirring. Eventually the mixture was evaporated to half its volume.11,14
Two treatments were performed, one using the crude enzyme extract (T1) and the other using the purified enzyme extract (T2), pH, temperature and reaction time remained similar. Table 1 shows a summary of treatments.
Total Reducing Sugar
1 mL of syrup before evaporation was taken. The aliquot was centrifuged to separate partially hydrolysed starch and maltose produced. Thereafter, 1 mL dinitrosalicylic acid (DNS) was added to the supernatant and kept in boiling water bath for 5 min.4 The test tubes were cooled to room temperature and 10 mL of distilled water was added with vortexing. Absorbance was measured at 540 nm using UV-Visible spectrophotometer (Raley UV-2100, China). Control experiment was performed with water and DNS. The amount of maltose produced was determined by standard maltose calibration curve (standards 10-50 g/L). Dextrose equivalent (DE) was calculated according to the formula:14
Determination of Soluble Solids (Sugar Brix)
A drop of 10 % v/v syrup was placed on the refractometer (HANNA Instruments, H196814). The refractometer was viewed, and the sugar level was read in brix degree (Brix°).3
Bromatological analysis of sweets potatoes and cassava
A sample of sweets potatoes and cassava were used to determine dry matter, crude fiber, humidity, and proteins, according to the methods described by the AOAC.15
Bromatological analysis of syrup
A sample of syrup (before evaporation) was used to determine the pH, dry matter, crude fiber, humidity, and proteins, according to the methods described by the AOAC.15
Qualitative determination of starch
1mL of syrup was dissolved with 15 mL of water. Three drops of Lugol reagent (5 % elemental iodine and 10 % potassium iodide) were added to solution, and vortex. The appearance of a blue-violet color indicates the presence of starch.16
Results and discussion
Bromatological analysis of sweets potatoes and cassava
When compared to other sources sweet potatoes have the major β-amylase enzyme. The main constituents of sweet potato are carbohydrates in particular starch. Besides the high starch content, sweet potato, amylase constitutes about 5 % of the total soluble protein of the tuberous root.17
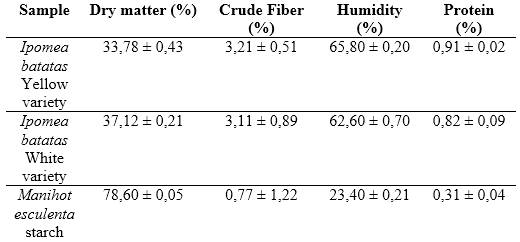
Considering that the biochemical composition of vegetables can vary according to climate and soil type, a bromatological analysis was performed to determine percentage of dry matter, crude fiber, moisture, and protein in three samples: white sweet potato, yellow sweet potato, and cassava starch. The results are shown in table 2.
Despite the high starch content in sweet potato (3,11-3,21 in the varieties studied), its extractability is very poor (≈ 70 % of the total starch) due to the latex in the roots and the pectin-hemicellulose complex preventing the free release of starch /18/. For this reason, it was decided to use sweet potato as a source of amylase and cassava as a source of starch.
Bromatological analysis allowed to determine which of the two sweet potato species has higher protein content, to be used as a source of β-amylase. In this study, the yellow variety with 0,91 % protein was selected. These results agree with those obtained by Ingabire et al., who report a higher percentage of proteins in the yellow variety.19
Quantification of protein in sweet potatoes extract
The main constituents of sweet potato are carbohydrates in particular starch. Sweet potato is one of the plant species with the highest content of amylases, with β-amylase being the majority.20
Several studies report the use of sprouted cereals as a source of amylases in obtaining syrup.3,8,18 A certain amount of the germinated mass is mixed with the starch, without knowing the real amount of amylase used, being necessary, in addition, to include a filtration step.
To know the real content of proteins in sweet potatoes extracts, a calibration curve was made, using BSA standards.
In our work, an aqueous extract of sweet potato in which amylases are found, was used. The amounts of proteins in the crude and purified extracts were 3,8 and 3,31 mg/mL, respectively, the lower amount of protein in the purified extract could be due to the loss of this component in the purification processes. Hesam et al., reported 3,2 mg/mL of protein in sweet potatoes grown in Iran.13 Starting from these results it is possible to calculate the amount of extract needed to hydrolyze a suspension to 20 % starch.9,15
Enzymatic hydrolysis of starch
In this work, β-amylase extracted from sweet potato of the yellow variety was used. Unlike cereals used as alternative sources of amylases, in which amylases are available only after germination; in the sweet potato β-amylase is synthesized during tuber development.2 Cereals are an important part of human and animal food, although they are an important source of amylases, the amount necessary for the hydrolysis of starch is not negligible, so it is necessary to look for other sources of the hydrolytic enzyme.
The enzymatic extract was added in two moments, at the beginning in the liquefaction stage and then two hours after starting the process, with this a greater availability of the enzyme is guaranteed. Ojewumi et al., obtained glucose syrup from cassava starch, achieving the best results when the germinated cereals as β-amylase source were added in two moments.3 On the other hand, by supplying the β-amylase extract in two steps, the mass is prevented from becoming very dense, remaining fluid after the second addition of the enzyme extract.
We worked in a range of two temperatures, in the first two hours the temperature gradually reached 50 °C, to complete the liquefaction, after the second the process temperature was maintained at 65 °C. According to Hesam et al., at this temperature the maximum activity of β-amylase is achieved.13
The pH of the mixture was maintained at 6, a value reported as optimal pH for the hydrolytic activity of β-amylase isolated from sweet potato.13) The adjustment of pH by means the addition of Ca(OH)2 is justified according to the increase in amylase activity in the presence of Ca2+ ions, based on its ability to interact with negatively charged amino acids such as aspartic and glutamic acids; this results in increased stabilisation of the active conformation of the enzyme. In addition to this interaction, calcium is known to play a role in binding the enzyme to the substrate.21
DE of the obtained syrup
To know the degree of hydrolysis achieved in cassava starch utilized as raw material, a calibration curve was developed using maltose as a standard because this is the main disaccharide formed when β-amylase is used.
From the calibration curve the content of reducing sugars in T1 and T2 was calculated. The values obtained are shown in table 3.
As shown in table 3, there were statistically significant differences regarding the content of reducing sugars in both treatments, being higher in T2; in which the purified enzyme extract was used. The quality of the syrups is determined by the DE factor, which indicates the percentage of reducing sugar with respect to the starting material for hydrolysis. In the T1 treatment the value of ED was 41,30, above 39, established as normal conversion, for the case of T2, an ED of 57,66 was reached, which represents a high conversion.22 On the other hand, ED values higher than 60, facilitate the crystallization of syrup at room temperature, and in industry fluid syrup is used.22
Other authors report for the enzymatic hydrolysis of cassava starch using cereals, ED values between 50-70 %, however, in the treatments studied it was not possible to exceed the value of 60 %.3 The different values of ED both treatments could be due to the degree of purity of the enzymatic extracts, in T1 the crude extract was used, however, the enzymatic extract used in T2 was purified by fractionation with ammonium sulfate and subsequently stabilized with a phosphate buffer.
The differences between our results and those reported in the literature, 3,13,19) could be related to the process of extraction of amylase, when using sprouted cereals, the enzyme is preserved in the grain, however this process is expensive, needs a stage of filtration and uses a raw material that is a fundamental component in the diet.
Proximal analysis of syrup
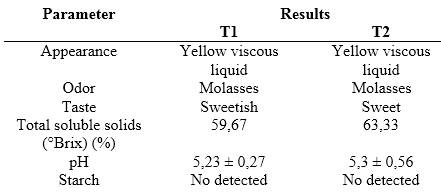
A proximal analysis of the syrup was performed to know the physicochemical and organoleptic properties of the same. The results are shown in table 4.
The color of the obtained syrup is yellow, unlike other studies that report a colorless syrup when cassava starch is used as raw material and plant extracts as sources of amylases.3) This difference may be due to the browning caused during the 8h of hydrolysis and the higher concentration of reducing sugars in T2.22 The smell of molasses indicates the presence of reducing sugars due to the hydrolysis of starch. The sweet taste is evidence of the formation of glucose and maltose, being sweeter the syrup obtained in T2, a result that coincides with its greater amount of reducing sugars and a higher percentage of ED. The increase of °Brix in T2 with respect to T1 could be related to the higher concentration of reducing sugars in this treatment. The pH values are similar in both treatments, which is influenced by the addition of buffer during the preparation of the enzymatic extracts. The absence of starch in the syrup obtained by both treatments is an indication that amylolytic enzymes involved with hydrolysis process of starch during mashing as contained in the extract might have done the process successfully.23 When starch concentrations greater than 20 % were used, starch was detected in the syrup.
The results obtained in this work, although they were carried out on a laboratory scale and are preliminary, constitute a starting point for using cassava starch and sweet potato extract as a source of β-amylase in the production of malt syrup.
Conclusions
β-amylase extracted from sweet potato can be used in the production of malt syrup from cassava starch. In both treatment (T1, T2), starch was not detected at the end of hydrolysis, indicating the effectiveness of the procedure used. The values of DE for the syrup obtained by means of T1 and T2 are within the reported parameters, however, when the purified enzyme extract (T2) was used, the best results were obtained.