Introduction
In the still close December 2019, an epidemic outbreak produced by a coronavirus, later named SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2), emerged among the population of the Chinese city of Wuhan. On February 11 of 2020, the World Health Organization (WHO) designated with the acronym COVID-19 (Coronavirus Disease of 2019) the disease caused by SARS-CoV-2.1 The wide spread of COVID-19 led the WHO to recognize it a pandemic on March 11, 2020.2
At a global scale, the efforts to halt the propagation of the still-young pandemic, and its very adverse sanitary, economic and social consequences, have been unsuccessful. As of March 28, 2021, the SARS-CoV-2 infection has reached 190 countries on all continents, leaving the unfortunate footprint of 126 372 442 infected people and 2 769 696 deaths.3
SARS-CoV-2 infection activates innate and adaptive immune responses that, in the most common and benign of evolutions, lead to the containment of the viral replication at the gateway of the host (the highest portions of the respiratory system), and in the least frequent and most unfavorable of the sequences, after allowing the virus to descend to the lower portions of the respiratory system, can stimulate an intense pulmonary inflammatory reaction that, leading to more severe complications, often ends in death.4 Generally, children, youth and healthy adults who infect with SARS-CoV-2 are at the most favorable end of that evolutionary spectrum. The opposite happens if the infection occurs in long-lived people and/or those suffering from chronic diseases.
Compared to economically developed regions with robust health systems, such as Europe and the United States, the severity of COVID-19 pandemic in Sub-Saharan Africa (SSA) has been relatively benign (Table).3 The poor economies, insufficient health services and high prevalence of comorbidities, mainly infectious diseases, characterizing that subcontinent, presaged a great sanitary catastrophe.5 Several reasons, non-exclusive, have been alluded to explain that unpredicted evolution in SSA: delay in systematic SARS-CoV-2 detection (included diagnostic test unavailability),5,6 quick implementation of lockdown measures (included physical distancing, enhanced hygiene and limitation to international air travel flows), (7 demographic composition with less people above 65 years old,8 genetic polymorphisms of the cell entry receptor for the SARS-CoV-2 (angiotensin converting enzyme 2, ACE-2),9 mutational variations of SARS-CoV-2 in relation with geographic settings,10 environmental temperature and humidity non-favorable for viral replication,11 cross-immunity to SARS-CoV-2,12 BCG vaccination policies,13,14 and, more recently, the anti-inflammatory component of the immune modulation triggered by helminth infections that are very prevalent there.15 Here, with the objective of obtaining a better comprehension on what has happened in SSA, we discuss the suggested influence of the systematic use of anti-malarial drugs on SARS-CoV-2 infection incidence and COVID-19 lethality in that region.
Table COVID-19 severity rates in Sub-Saharan Africa, Europe and United States as of March 28, 2021
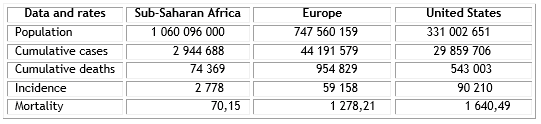
Confirmed Case: person positive by Polymerase Chain Reaction (PCR) test for SARS-CoV-2.
COVID-19 death: a COVID-19 death is defined as a death resulting from a clinically compatible illness in a probable or confirmed COVID-19 case, unless there is a clear alternative cause of death that cannot be related to COVID-19 disease (e.g. trauma). There should be no period of complete recovery between the illness and death.
Incidence: Cumulative cases per 1 million populations.
Mortality: Cumulative deaths per 1 million populations.
Source: World Health Organization. Coronavirus disease 2019 (COVID-19): Weekly Epidemiological Update, March 28, 2021. Geneva: WHO, 2021.3
COVID-19, Sub-Saharan Africa and anti-malarial drugs
As sanitary problem, malaria is declining globally; however, it is still a major challenge for the public health and the socio-economic development in some regions of the world, particularly in SSA. During 2017, there were an estimated 219 million malaria cases and 435 000 deaths worldwide. From this, 92% of the cases occurred in SSA.16 Chloroquine (CQ) and its derivative molecule hydroxychloroquine (HCQ) were introduced for the treatment and prophylaxis of malaria in 1947 and 1955, respectively. Since then, and until the beginning of the present century, CQ and HCQ were the drugs most used for reducing the incidence and severity of Plasmodium infection.17
CQ and HCQ have demonstrated in vitro antiviral and anti-inflammatory activities.18 At least, four mechanisms have been proposed to explained those activities: (i) CQ may reduce glycosylation of ACE2, thus preventing virus attachment process to the host cell,19 (ii) CQ accumulates in lysosomes, which may interrupt the regular course of lysosome-endosome fusion, thereby inhibiting the release of the viral contents,20 (iii) CQ increases lysosome pH, thus impairing macrophage antigen processing [21],21) and (iv) CQ blocks the production of IL-6 and other pro-inflammatory cytokines, which are key mediators of acute respiratory distress syndrome.22 Besides the attractive rational of their antiviral and anti-inflammatory activities, CQ/HCQ have an additional advantage, mainly for low income countries: their immediate availability at a very low cost.23
Barely based on the in vitro findings of antiviral and immunomodulatory activities, and pushed by the desperate need for an effective handling of the rapidly expanding viruses, CQ and HCQ have been used for the treatment of COVID-19 since the beginning of the pandemic. Unfortunately, the overall evaluation of the most recent evidence shows that, at least at the doses employed, those drugs are not effective in reducing the viral load, decreasing the need for respiratory support or improving COVID-19 patients survival.24 Certainly, there is no evidence of CQ and HCQ having successfully controlled any acute viral infection in human beings.25
Apart from the use of CQ and HCQ in the treatment of COVID-19, it has been suggested that the systematic anti-malarial use of those drugs in SSA may represent a form of unintentional pre-exposure prophylaxis against SARS-CoV-2 infection, which potentially slows down the spread of the epidemic and attenuates its severity there.6,26,27,28 Three types of arguments that could sustain that suggestion have been mentioned: (i) the historical data related to SARS-CoV epidemics, that reveal some efficacy of those drugs against SARS-CoV,29 (ii) the results of a retrospective observational study performed on healthcare workers in India, suggesting that consumption of 4 or more weekly maintenance doses of HCQ for prophylaxis was associated with lower risk of contracting SARS-CoV-2 infection,30 and (iii) the comparison of COVID-19 epidemiological rates in sceneries with and without the use of CQ/HCQ;27,31 for instance, an appraisal found a lesser lethality rates in a group of countries that use or produce CQ or HCQ on a massive scale during COVID-19 pandemic compared to a second group of countries that did not during the same period.31
However, an increasing number of solid reasons refute the occurrence of an effective unintentional pre-exposure prophylaxis of SARS-CoV-2 infection by the systematic anti-malarial use of CQ/HCQ in SSA. First, consequence of the emergence of Plasmodium 4-aminoquinoline resistance strains in all endemic regions, including SSA, CQ/HCQ have been replaced by artemisinin based combination therapy (ACT) for the prophylaxis and treatment of malaria in SSA about 2005.32
Second, and referring to the results of other type of unintentional pre-exposure prophylaxis of SARS-CoV-2 infection by the systematic use of CQ/HCQ, it was published a large retrospective study that took the advantage of a setting in which a specific group of patients has been receiving HCQ over several months to years before the novel coronavirus emerges among the analyzed population. In that study, the proportion of patients with laboratory-confirmed SARS-CoV-2 infection did not differ between people with rheumatologic conditions (rheumatoid arthritis, systemic lupus erythematous, and other associated autoimmune disorders) who received HCQ and those with similar conditions who did not received the drug, suggesting that HCQ does not prevent SARS-CoV-2 infection.33
Third, recent and better designed studies have demonstrated that the intentional pre-exposure prophylactic use of CQ/HCQ does not confer protection against SARS-CoV-2 infection.24,34
In relation with other antimalarial drugs, it should be mentioned the recent demonstration of the in vitro inhibition of SARS-CoV-2 replication by mefloquine-artesunate.32 Although in vitro activity is not necessarily linked to clinical efficacy, the establishment of in vitro effectiveness of ACT against SARS-CoV-2 may provide some information concerning the possible involvement of this type of antimalarial drug in the lower spread and severity of COVID-19 pandemic in SSA. In this respect, clinical and epidemiological trials are required.
Conclusions
Taken together the arguments discussed here, the evidence supporting the influence of the systematic use of anti-malarial drugs, in particular CQ and HCQ, on the low SARS-CoV-2 infection incidence and COVID-19 lethality in SSA appears very weak. A myriad of additional factors, some of them mentioned above, may be related with the unexpected evolution of COVID-19 pandemic in that impoverished region. Looking ahead, those factors merit to be studied in depth for reaching a rational implementation of control actions in those countries, including the administration of COVID-19 vaccines.














