Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.27 n.2 La Habana abr.-jun. 2010
REVIEW
Heberprot-P: experimental background and pharmacological bases
Heberprot-P: antecedentes experimentales y bases farmacológicas
Jorge Berlanga
División de Farmacéuticos, Investigaciones Biomédicas, Centro de Ingeniería Genética y Biotecnología, CIGB Ave. 31 entre 158 y 190, Cubanacán, Playa, CP 10 600, Ciudad de La Habana, Cuba
ABSTRACT
Heberprot-P is a novel drug intended to solve an unmet medical need: to heal high -grade, poor-prognostic ulcers which affect lower limbs of diabetic patients. The human recombinant epidermal growth factor (hrEGF) is the active pharmaceutical ingredient of Heberprot-P. EGF is a highly evolutionarily conserved polypeptide playing a significant role on the intra and extra-uterine life in mammals. Based on the early findings of its epitheliotropic and mitogenic effects, it was prematurely intended as healing agent for problematic wounds. Our Center for Genetic Engineering and Biotechnology manufactures (EGF) since 1988. About 1991, we unleashed an intense experimental research program on in vivo systems which somewhat mirrored a variety of human pathological conditions . Those studies accounted for the identification of novel pharmacological effects associated to the systemic or parenteral administration of EGF. Henceforth it enabled us to envision new therapeutic indications to treat processes requiring cytoprotective effects. We had demonstrated since 1995, that the local infiltration of EGF in the hindlimbs of rats, mitigated the degenerative process on peripheral nerves and soft-tissues undergoing the consecuenses of denervation. Further studies evidenced the ability of EGF to rescue tissues and organs from death by ischemia/reperfusion events, and also in models of multiorgan damage under acute preconditioning or therapeutic schedules. During that decade, we demonstrated the need to preserve EGF from the action of proteases released in full-thickness controlled wounds. All these aspects were pieces of knowledge supporting the hypothesis on the beneficial effect of the intralesional infiltration of EGF to rescue and perpetuate cells in diabetic ulcers ensuring an appropriate local bioavailability.
Keywords: Epidermal growth factor, Heberprot-P, wound healing, diabetic foot ulcer, cytoprotective, preclinical, toxicology
RESUMEN
El Heberprot-P constituye un novedoso medicamento encaminado a solucionar una necesidad médica no cubierta: la cicatrización de la ulcera de alto grado de miembros inferiores de pacientes diabéticos. Su ingrediente farmacéutico activo es el factor de crecimiento epidérmico humano recombinante (EGF). Este polipétido ha sido conservado a lo largo del proceso evolutivo y desempeña un importante papel en la vida intra y extrauterina de los mamíferos. La identificación preliminar de sus efectos epiteliotropos y mitogénicos condujo a su prematura evaluación como cicatrizante. Nuestra institución el Centro de Ingeniería Genética y Biotecnología, produce EGF humano recombinante desde 1988. A partir de 1991 desarrollamos un intenso programa de experimentos sobre sistemas in vivo que recreaban diversas patologías humanas. Estos estudios permitieron la identificación de novedosos efectos farmacológicos asociados a la administración sistémica o parenteral del EGF; y en consecuencia sugerir nuevas indicaciones terapéuticas para procesos tributarios de efecto cito-protector. Desde 1995 habíamos demostrado que el tratamiento infiltrativo local con EGF en las extremidades posteriores de ratas, mitigaba los efectos degenerativos en nervios periféricos así como los efectos necrogénicos sobre piel y planos blandos denervados. Estudios ulteriores evidenciaron la capacidad del EGF para rescatar órganos y tejidos de la muerte por isquemia/reperfusión y modelos de daño multi-orgánico bajo esquemas precondicionantes o terapéuticos agudos. Durante esa década demostramos la necesidad de preservar el EGF ante el efecto de proteasas derivadas de heridas controladas agudas. Todos estos aspectos constituyeron piezas de conocimiento que nutrieron la hipótesis acerca del efecto favorable de la infiltración intralesional del EGF en las ulceras, para rescatar y perpetuar células bajo adecuada biodisponibilidad local del EGF.
Palabras clave: Factor de crecimiento epidérmico, Heberprot-P, cicatrización, úlcera del pie diabético, citoprotector, preclínica, toxicología
INTRODUCTION
Heberprot-P is a novel injectable medicine developed at the Center for Genetic Engineering and Biotechnology of Havana (CIGB) and as such, a product of Cuban biotechnology–as was dreamed and created by Fidel Castro more than 20 years ago–. This medicine contains the human recombinant Epidermal Growth Factor (EGF) as the Active Pharmaceutical Ingredient. Its therapeutic properties, mode of application and niche for indications are unique in the world. No other specific medication exists today that is able to stimulate and sustain the healing process of wounds and complex ulcers on the lower limbs at terminal stages in diabetic patients. Unique is also its history, including the enormous efforts made to have this medicine reach all Cubans needing it. In short, while this medicine draws on the biological properties of EGF and the opportunities offered by its application method, it is able to satisfy a medical need not previously covered in the world.
EPIDERMAL GROWTH FACTOR. THE ACTIVE PHARMACEUTICAL INGREDIENT OF HEBERPROT-P
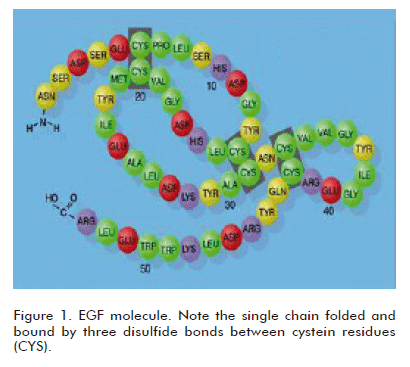
EGF is an ancient polypeptide, conserved throughout evolution, which is present in many animal species and with a remarkably preserved structure having a high interspecies homology, and therefore showing cross-species biological activity. This latter property has been a corner stone in developing a vast series of pharmacological and toxicological experimental studies using the human natural or recombinant EGF. A large number of studies and reports in the literature demonstrate that the human EGF binds to the receptor expressed by the cells of laboratory animals (1). In fact, the discovery of EGF by Stanley Cohen was supported by the identification of its epitheliotropic effects in different laboratory rodent species, by administering a semi-pure preparation extracted from their salivary glands (2). Undoubtedly, the phylogenetic conservation of EGF in animals and man, and its ubiquitous distribution in tissues and organic fluids, suggest the existence of a relevant physiological role in the homeostatic preservation of epithelial cell populations in the form of mucosae or glands. From the chemical viewpoint, EGF is a relatively small molecule of only 53 amino acids, although smaller and larger molecular species or isoforms having physiological relevance have been described. It is a self-folded single chain with a loop formed by three disulfide bonds between cystein residues (Figure 1).
As described for other growth factors, the biological activities of EGF are induced after binding to the receptor molecule, which is extensively distributed in many mesenchymal and epithelial tissues. The receptor, known as HER-1, is a trans-membrane protein that has a terminal intra-cytoplasmic domain with a tyrosine kinase activity, significantly involved in signal transduction through several occasionally convergent and redundant pathways. The systemic administration of EGF at supra-physiological concentrations is able to induce biological responses that were formerly assumed as redundant effects. However, in the last 20 years, transgenic mice or those genetically silenced for specific growth factors have helped unravel its subtle physiology and biochemistry at the cellular level.
Almost since its discovery and identification, researchers have evaluated the effects of EGF extracted and purified from salivary glands in experimental systems of rabbit corneal burn. Preliminary findings at that time demonstrated that the local administration of EGF stimulated the migration and proliferation of the corneal epithelium, helping the wounds heal faster than normal. As early as the beginning of the 1960’s, it was known that the healing effect was due to its mitogenic action on epithelial cells and fibroblasts. Using cell culture models it was demonstrated that the presence of EGF in cultures of fibroblasts, endothelial cells and keratinocytes favored their proliferation and, in the latter, their migration also. In other words, the main cell types responsible for skin wound healing expressed the EGF receptor and were stimulated by its presence. All the pieces of knowledge obtained up to that moment from the rabbit corneal burn in vivo model and from in vitro experimental systems, stimulated the design of two excellent experiments in mice. They included the surgical removal of salivary glands from a group of wounded mice, and the exogenous application of saliva to the wounds. It was conclusively demonstrated that animals lacking salivary glands, and therefore unable to lick their wounds, showed significantly deficient wound healing. On the other hand, the exogenous application of saliva accelerated the healing process. The role of EGF from saliva in the skin healing process was thus demonstrated.
Almost 50 years of biological research using EGF in different systems and experimental contexts make it possible to summarize its general functions as a:
- Mitogenic agent: It controls and stimulates cell proliferation, particularly in epithelial tissue cell lines.
- Motogenic agent: It controls and stimulates cell migration.
- Inducing agent for cell differentiation: It promotes the production of a defined or differentiated phenotype in undifferentiated or pre-differentiated cells.
- Cyto-protective agent: It stimulates cell survival in episodes or insults that would otherwise be lethal.
The systemic administration of EGF at supra-physiological concentrations is able to induce biological responses as those described above and this can therefore result in pharmacological actions through the extension or enhancement of the biological response (3).
CUBA PRODUCES THE HUMAN RECOMBINANT EGF
The CIGB of Havana has produced the human recombinant EGF since 1988 as a mixture of isoforms of 51 and 52 amino acids. At that time, our country was one of the pioneers in obtaining this protein through genetic engineering techniques. This product has been subjected to an extensive series of pre-clinical, experimental toxicological and pharmacological studies. Undoubtedly, it is the most pre-clinically investigated Active Pharmaceutical Ingredient of the CIGB. Many pharmacological studies have being carried out by the CIGB and other Cuban institutions with this product. Other studies have been performed in cooperation with internationally relevant foreign scientific research groups. The EGF produced in Cuba was examined and characterized by a group of British researchers, who demonstrated its high quality based on its physicochemical integrity, purity and biological potency. Those results allowed and facilitated its use in a successful clinical trial in the United Kingdom (4, 5). Cuba had produced Hebermin for the last 20 years, a pharmaceutical semi-solid EGF formulation used to stimulate wound healing in acute cutaneous lesions.
BACKGROUND AND EXPERIMENTAL STUDIES. PRE-CLINICAL PHARMACOLOGICAL BASIS FOR EGF
The experimental models used in EGF pharmacological studies have been exploited extensively and are fully valid. These biomodels ad integrum have made it possible to discover new pharmacological effects of this agent, which consequently suggest new therapeutic indications for the experimentally represented conditions. The animal models used are confirmed to be solid and useful by their repeatability and reproducibility. In the 1990’s, the development of pre-clinical pharmacology for the systemic administration of EGF was academically validated in Cuba and other countries. Here we describe the sequence of studies carried out at the CIGB.
The cyto-protective and gastro-protective potentials of EGF, particularly the latter, were studied in 1992 using an experimental system that is extensively employed in canonical pharmacology. This system is based on preventing and attenuating gastric damage in rats exposed to the oral instillation of ethanol. EGF was administered by the oral route under pre-conditioning prophylactic schedules and a therapeutic schedule. This simple assay was the first of a series of studies aimed to evaluate the cyto-protective effects of EGF. Under these experimental conditions, EGF showed its capacity to prevent and reduce gastric damage in a dose-dependent way. Interestingly, the preventive effect, associated to the prophylactic treatment showed clear evidence of the protective potency of EGF in an epithelial substrate challenged by a necrogenic agent. In other words, EGF seemed to activate or pre-condition cellular processes opposing the lethal effects of an external chemical agent. This information was never published but it has contributed to a more complete documentation within our sanitary records.
Inspired by the curiosity and enthusiasm caused by the above results, we decided to evaluate, for the first time, the effect of the systemic injection of EGF. We hoped to learn about its protective effect in a clinically and pathologically relevant animal model, and in tissues unrelated to the upper structures of the digestive system. At that moment, the literature was full of demonstrations in rats reproducing Curling’s ulcers. Therefore, the examination of the neuroprotective effect of the perilesional injection of EGF in rats that had been subjected to total transversal axotomy of the sciatic nerve in the coxofemoral area was determined. The morpho-functional regenerative response of the axon was studied, as well as the synthesis of myelin by the nerve and its cells. Two independent and extemporary studies demonstrated that this treatment produced the following effects:
a) It significantly favored the recovery of the motor nerve conduction 60 days after provoking the trauma. This was a delayed effect, since EGF was administered for only 20 days.
b) It favored axonal and myelin recovery. It prevented or reduced the changes suggesting a within axonal degeneration. EGF also favored, in a dose-dependent way, the survival of myelin producing cells near the sectioned area, contributing to a more physiological re-myelination.
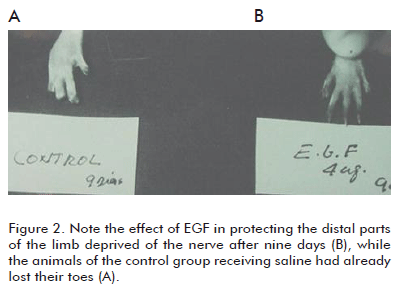
c) It prevented or significantly delayed the onset of trophic changes of the soft tissues of the limbs (skin and related structures). The presence of plantar ulcers and toe necrosis was delayed (Figure 2).
The cyto-protective effects of EGF on the nervous tissues were evident, a conservatively novel finding at that time. However, the recurring observation that EGF could protect the soft peripheral tissues in a denervated limb was completely new. The prevention of hair loss under these conditions was perhaps the most immediate response to the EGF infiltration treatment. The fact that EGF showed an anti-necrogenic effect under these experimental conditions, suggested an unusual cyto-protective capacity with a high potential for therapeutic use, according to the physiopathological relevance of the model implemented for the lesions. The sectioning of the sciatic nerve implied a drastic deterioration of the vascular neural tone and of the vasomotor reserve, which unquestionably will have negative consequences in the irrigation of oxygenated blood to the skin, as well as in its venous return. In other words, the possibility of establishing the pharmacological management of a tissue hypo-perfusion process of neurogenic origin had begun (6, 7).
Other experiments followed during one decade that were more focused on studying the potential cytoprotective effect of EGF in experimental ischemic episodes, when administered by the parenteral route and following pharmacological preconditioning schedules or therapeutic schedules of multiple injections. Hence, the evaluation of the hepato-protective effect of EGF was started in an experimental system involving its anti-inflammatory and anti-oxidant induction effects in order to rescue the hepatocytes. The EGF was also administered through the intra-peritoneal route in rats, 30 minutes before being challenged with hepatotoxic carbon tetra-chloride. The experiment demonstrated the hepato-protective action of EGF through a substantial reduction of parenchymal necrosis in this organ, compared to the animals of the control group receiving saline (8). The effects were dose-dependent, and were a first demonstration of the EGF-induced protection against a cytotoxic agent having a damaging mechanism involving lipid peroxidation. An ischemia/reperfusion experiment was carried out to demonstrate in a more realistic and clinically relevant setting the antioxidant defense recently described. The most appropriate and available model at that time was the bilateral renal ischemia, which was reproduced successfully by the late Professor Ernesto Barber. Depending on the dose, the preconditioning treatment prevented the morphological and functional deterioration of the kidney tissue. The animals treated with EGF showed a significant reduction in the levels of all the oxidative stress markers studied, as well as in the activity of the PLA-A2 as a precursor of pro-inflammatory mediators and agents related to the spasm of kidney vessels (9). Figure 3 shows the protective effects of the treatment.
Our group carried out a second ischemia/reperfusion experiment. It was conceived as the temporary occlusion of the upper mesenteric artery that irrigates the jejunum and ileum segments. In addition to demonstrating the cyto-protective effect of EGF against ischemia, this experiment would be somewhat of a theoretical introduction to models of multiple organ damage (MOD). Although it was not the main objective, this experiment in itself produced an excellent reproduction of MOD. It is known that a systemic inflammatory response syndrome (SIRS) established during the reperfusion phase of the small intestine, is characterized by high serum levels of the tumor necrosis factor (TNF-α) that is morphologically expressed in several organs, such as in the lungs by an edema of capillary permeability at the septum (similar to the Adult Respiratory Distress Syndrome, ARDS) and in the kidneys by acute tubular necrosis.
There is a notably dramatic increase in the levels of malondialdehyde (MDA) as a marker of lipid peroxidation in different internal organs, as well as of myeloperoxidase (MPO) as an inflammation marker of activated neutrophils infiltrated within the parenchyma of tissues. Intestinal damage, such as hemorrhage and partial infarctions, was significantly prevented in animals receiving a single prophylactic injection of EGF (Figure 4). Kidney and lung damage were also significantly reduced. From a biochemical point of view, it was demonstrated that the treatment with the EGF reduced the formation of oxygen reactive species, as well as the activity of the enzyme MPO. An interesting finding was that circulating TNF-α levels substantially decreased in the groups treated with EGF (10). To conclude the investigation we just had to establish an MOD model and assess the EGF in a lethal pathological and progressive organic deterioration substrate.
The capacity of EGF to establish a systemic and simultaneous cyto-protective effect in various internal epithelial organs was examined, after demonstrating that a single preconditioning EGF injection generated dose-dependent entero-, hepato- and nephroprotective effects. For that purpose, a sterile MOD model induced by severe burns was established in mice and rats receiving hypodermic lesions in 25 to 30% of their body surface (11). Animals received a single EGF injection, 30 minutes before burning. The results of the experiment were: 1) a significant reduction in cumulative mortality in three independent experiments, 2) a decrease in kidney damage, and 3) decreased gastrointestinal damage. Figure 5 shows the substantial difference of mucosal integrity at the jejunum and ileum between animals treated with EGF and those receiving only normal saline.This was the first study demonstrating the capacity of a single and prophylactic injection of EGF to induce simultaneous protection in several organs, an effect that was not disrupted under such a severe stress as burns affecting the internal organs. In a second demonstration of MOD induced by a chemical substance (12), a prophylactic or therapeutic intervention with EGF significantly increased animal survival, preventing kidney, liver and intestinal damage and also reducing the levels of numerous biochemical markers indicating functional alterations in different organs.
In a later study, we were to assess the intestinal response to the continuous exposure to EGF. Until then it was known that EGF and other growth factors from that same family exerted a trophic effect on the mucosa of the gastrointestinal system, although the bases mediating that response had not been yet described. One of the purposes of this study was to give the scientific support to the systemic administration of EGF in order to regenerate intestinal mass and tissues. The continuous infusion of EGF (60 μg/kg per day) was uniformly ensured through the use of subcutaneous osmotic mini-pumps in periods between days 1 to 14. The study made it possible to learn that both cell proliferation in the crypt and its fission were independent but complementary mechanisms for the trophic effect of EGF in this system (13). It was demonstrated that the colon segment is particularly sensitive to EGF, where an increase and later regression of the index of crypt fission was observed, suggesting an endogenous mechanism for controlling tissue growth. On the other hand, this study demonstrates the potent effect of EGF on the growth of intestinal villi in different segments of the small intestine of enterally-fed animals.
Figure 6 shows the effect of EGF on the growth of villi and crypt fission a few days after starting the treatment. Compare the difference with animals receiving normal saline. This study established the potential bases for EGF treatment in pathological processes requiring the regeneration of intestinal masses and tissues, and the correction of villi atrophy or flattening, thus being able to increase its absorption area.
Studies in which EGF was administered together with the Keratinocyte Growth Factor (KGF) showed that intestinal atrophy can be reverted by treating with EGF, and that this response can be enhanced by the concurrent administration of KGF (14). This experiment confirmed previous results of the trophic effect of EGF on the gastrointestinal mucosa. This and subsequent studies form part of a series of experiments on the effect of EGF on the digestive system, thus providing new theoretical support to correct atrophy and stimulate the healing of the intestinal mucosa, and to improve the condition of the organism with a malabsorption syndrome.
Finally, the most recent study of this series (15), shows that a single pre-conditioning administration of EGF in rats, alone or combined with the growth hormone-releasing peptide 6 (GHRP-6), exerts a potent protective effect on rat liver parenchyma exposed for 120 min to ischemia and after 3 hours of reperfusion. Damage due to the activation of the pro-inflammatory cascade became significantly attenuated in distal organs such as the lungs, small intestine and kidneys. This confirms the systemic cyto-protective effect of EGF (Figure 7).
After working several years with EGF in different experimental biological sys-tems, it may be concluded that there are two basic pharmacological effects that are induced by administering EGF at supra-physiological concentrations: 1) cyto-protection, which is rapidly established, even after just a single dose, and 2) trophic- repairing effects, which appear after repeated and more or less prolonged administrations. Considering the risk-benefit ratio, the substantial advantages and virtues of using EGF to treat niches that have been unattended in clinical medicine have been clearly explained. The rationality of the infiltrating treatment of diabetic foot ulcers is supported by the findings of our group showing a reduced local bioavailability of this factor when topically applied on acute and controlled total thickness wounds.
PRE-CLINICAL TOXICOLOGY STUDIES. CONSIDERATIONS ON THE TRANSFORMING POTENTIAL OF EGF
As previously mentioned, EGF has been submitted to a wide series of pre-clinical safety studies with repeated administration long-term schedules in different laboratory animal species. Genotoxicity studies have also shown negative results. Until now, the evidence shows that EGF does not trigger the process of a malignant transformation of normal cells under the conditions tested and even after its repeated and exogenous administration (16). However, classical experiments clearly indicate that EGF promotes chemical and viral carcinogenesis (17) in animals and cells, respectively. In other words, cells in cultures or within the body of laboratory animals, which were exposed to viruses or carcinogenic initiating substances, can become more easily tumorigenic after EGF treatment. It is also well known that in several types of human epithelial tumors there is an over-expression of the EGF receptor and its signaling system is over-amplified (18).
The first systemic intervention with EGF was carried out, as far as we know, in 1975, in patients with Zollinger-Ellison syndrome. Other severe pathological processes of the gastrointestinal tract have also been treated with the systemic administration of EGF (19). Similar studies are being carried out throughout the world (20). On the other hand, the most recent clinical trials were conducted by our group, infiltrating EGF locally to diabetic foot ulcers that were refractory to treatment (21). Throughout these years, no carcinogenic episodes have been observed in any of the patients treated with these repetitive therapeutic interventions using EGF at supra-physiological concentrations and with any of the administration routes used.
Is obvious to point out that EGF therapy, or that of any other growth factor, is strictly contraindicated in patients having malignant tumors or pre-malignant lesions. The administration of the EGF or any other growth factor must always be carried out with the careful selection and inclusion of patients, based on their personal and family backgrounds, and also considering the risk-benefit ratio. In fact, this rule is applied in clinical practice for many authorized and internationally prescribed drugs.
Biological and clinical evidence that support the criterion that EGF does not seem to be an initiator of cell transformation toward malignancy is summarized below.
1. EGF is not genotoxic or mutagenic according to results of internationally established evaluations. It does not seem to modify cell stability (22).
2. The in vitro and in vivo pre-clinical systems indicate a tumor-promoting activity of EGF in cellular niches previously “initiated” with chemical or viral carcinogens. The tumor-promoting activity of EGF has not always been reproduced in vivo (23).
3. The exogenous administration of EGF, of the transforming growth factor alpha and of other growth factors, has been shown to be insufficient in inducing tumors. It has been demonstrated that the inadequate exposure to EGF of transgenic mice predisposes the target tissue to cancer, and EGF could be a necessary, but insufficient factor for cell transformation (24).
4. In other EGF transgenic models, over-exposure to EGF was associated only to delayed somatic growth (25) and to the enhanced adaptability of the target cells after small bowel resection (26).
5. Hyperplastic changes in animal models mediated by a continuous exo-genous exposure to EGF seem to be self-limited. The trophic changes mediated by EGF in animal models are reversible and depend on the dose, frequency, species and sex in susceptible tissues (27-30).
6. It is known that the healing process comprises a set of complex convergent events, such as cell migration, differentiation and division, all of which condition the promotion of tumor growth (31). There are, however, no reports of oncogenic induction in patients receiving EGF or any other topically-administered growth factor. On the contrary, the topical administration of EGF has been reported as radioprotective in patients with a histological diagnosis of skin carcinoma submitted to radiotherapy (32).
7. Pharmacokinetic and bio-distribution profiles of EGF in animal models and in man have shown its short half-life with a rapid elimination and no accumulation, thus excluding the concept of the continuous/ long-term activation of the receptor.
CONCLUSIONS
EGF has proved to be clinically safe up to now, and its administration may be useful in certain clinical processes that are refractory to treatment and/or critical conditions. These are the niches in which its application is adequately adjusted to ethical and therapeutic standards when following a risk-benefit ratio analysis for each specific case.
REFERENCES
1. Brown KD. The epidermal growth factor/ transforming growth factor-a and their receptors. Eur J Gastroenterol Hepatol 1995;7:914-22.
2. Precocious newborn mice and Epidermal Growth Factor: the work of Stanley Cohen. Papers in a series reprinted to celebrate the centenary of the JBC in 2005. J Biol Chem 2006;281:10-1.
3. Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, van Bergen en Hengowen PB. The epidermal growth factor. Cell Biol Int 1995;19:413-30.
4. Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med 2003;349:350-7.
5. Calnan DP, Fagbemi A, Berlanga- Acosta J, Marchbank T, Sizer T, Lakhoo K, et al. Potency and stability of C truncated human epidermal growth factor. Gut 2000; 47:622-7.
6. Prats P, Castañeda L, Berlanga J, Falcón V, Rodríguez V, Suarez-Muria C. El factor de crecimiento epidérmico en lesiones del sistema nervioso periférico. Rev Mex Cienc Farm 1998;29:17-23.
7. Prats P, Castañeda L, Falcón V, Ortega R, de la Rosa MC, Menéndez I, et al. Efecto del Factor de Crecimiento Epidérmico sobre la regeneración del nervio ciático transectado en ratas. Biotecnol Apl 1998; 15:237-41.
8. Berlanga J, Caballero ME, Ramírez D, Torres A, Valenzuela C, Lodos J, et al. Epidermal growth factor protects against carbon tetrachloride-induced hepatic injury. Clinical Science (Lond) 1998;94: 219-23.
9. Caballero ME, Calunga J, Barber E, Cruz E, López-Saura P, Boix E, et al. Epidermal Growth Factor-mediated prevention of renal ischemia/reperfusion injury. Biotecnol Apl 2000;17:161-5.
10. Berlanga J, Prats P, Remírez D, González R, Lopez-Saura P, Aguiar J. Prophylactic use of epidermal growth factor reduces ischemia/ reperfusion intestinal damage. Am J Pathol 2002;161:373-9.
11. Berlanga J, Lodos J, López-Saura P. Attenuation of internal organs damages by exogenously administered epidermal growth factor (EGF) in burned rodents. Burns 2002;28:435-42.
12. Caballero ME, Berlanga J, Ramírez D, Lopez-Saura P, González R, Floyd DN. Epidermal growth factor reduces multiorgan failure induced by thioacetamide. Gut 2001;48:34-40.
13. Berlanga-Acosta J, Playford RJ, Mandir N, Goodlad RA. Gastrointestinal cell proliferation and crypt fission are separate but complementary means of increasing tissue mass following infusion of epidermal growth factor in rats. Gut 2001;48:803-7.
14. Sasaki M, FitzGerald AJ, Mandir N, Berlanga-Acosta J, Goodlad RA. Keratinocyte growth factor and epidermal growth factor can reverse the intestinal atrophy associated with elemental diets in mice. Exp Physiol 2003;88:261-7.
15. Cibrian D, Ajamieh H, Berlanga J, Leon OS, Alba JS, Kim MJ, et al. Use of growthhormone- releasing peptide-6 (GHRP-6) for the prevention of multiple organ failure. Clin Sci (Lond) 2006;110:563-73.
16. Berlanga J, Álvarez S, de la Fuente J, López-Saura P. Considerations on the transforming potential of epidermal growth factor. Biotecnol Apl 1998;(15):65-70.
17. Stoscheck CM, King LE. Role of Epidermal Growth Factor in carcinogenesis. Cancer Res 1986;46:1030-7.
18. Arteaga CL. Epidermal Growth Factor receptor dependence in human tumors: more than just expression? Oncologist 2002;7(Suppl 4):31-9.
19. Guglietta A, Sullivan PB. Clinical applications of epidermal growth factor. Eur J Gastroenterol Hepatol 1995;7:945-50.
20. Sullivan PB, Lewindon PJ, Cheng C, Lenehan PF, Kuo BS, Haskins JR, et al. Intestinal mucosa remodeling by recombinant human epidermal growth factor (1-48) in neonates with severe necrotizing enterocolitis. J Pediatr Surg 2007;42(3):462-9.
21. Fernández-Montequín JI, Infante-Cristiá E, Valenzuela-Silva C, Franco-Pérez N, Savigne- Gutierrez W, Artaza-Sanz H, et al. Intralesional injections of Citoprot-P (recombinant human epidermal growth factor) in advanced diabetic foot ulcers with risk of amputation. Int Wound J 2007;4(4):333-43.
22. Maraschin R, Bussi R, Conz A, Luciana O, Pirovano R, Nyska A. Toxicological evaluation of u-hEGF. Toxicol Pathol 1995;23(3):356- 66.
23. Lu Xia, Yao-Zong Yuan, Chun-Di Xu, Yong-Pin Zhang, Ming-Ming Qiao, Jia-Xu Xu. Effects of epidermal growth factor on the growth of human gastric cancer cell and the implanted tumor of nude mice. World J Gastroenterol 2002;8(3):455-8.
24. Borlak J, Meier T, Halter R, Spanel R, Spanel-Borowski K. Epidermal growth factorinduced hepatocellular carcinomas: gene expression profiles in precursor lesions, early stage and solitary tumors. Oncogene 2005;24(11):1809-19.
25. Chan SY, Wong RWC. Expression of Epidermal Growth Factor in transgenic mice causes growth retardation. J Biol Chem 2000;275:38693-8.
26. Erwin CR, Helmrath MA, Shin CE, Falcone RA Jr, Stern LE, Warner BW. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol 1999;277(3 Pt 1):G533-40.
27. Vinter-Jensen L, Smerup M, Kissmeyer- Nielsen P, Poulsen SS. Chronic systemic treatment with epidermal growth factor in the rat increases the mucosal surface of the small intestine. Regul Pept 1995;60(2-3):117-24.
28. Vinter-Jensen L, Juhl CO, Poulsen SS, Djurhuus JC, Dajani EZ, Nexo E. Chronic administration of epidermal growth factor to pigs induce growth especially of the urinary tract with accumulation of epithelial glycoconjugates. Lab Invest 1995;73(6):788-93.
29. Juhl CO, Vinter-Jensen L, Poulsen SS, Orntoft RF, Dajani EZ. Chronic treatment with epidermal growth factor causes esophageal epithelial hyperplasia in pigs and rats. Dig Dis Sci 1995;40(12):2717-2723.
30. Christiansen JJ, Vinter-Jensen L, Nielsen K. Systemic treatment in the rat with epidermal growth factor causes polycystic growth of ovaries. APMIS 1996;104:147-52.
31. Berlanga-Acosta J, Gavilondo-Cowley J, López-Saura P; González-López T, Castro-Santana M, et al. Epidermal Growth Factor (EGF) in clinical practice- A review of its biological actions, clinical indications and safety implications. Int Wound J 2009;6:331-46.
32. Alert J, Rodríguez J, Lombardero J, Pérez R. Acción radioprotectora local del factor de crecimiento epidérmico humano recombinante: reporte preliminar. Interferón Biotecnol 1989;6:62-6.
Received in July, 2009.
Accepted for publication in November, 2009.
Jorge Berlanga, Centro de Ingeniería Genética y Biotecnología, Investigaciones Biomédicas, División de Farmacéuticos, Ave. 31 entre 158 y 190, Cubanacán, Playa, CP 10 600, Ciudad de La Habana, Cuba. E-mail: jorge.berlanga@cigb.edu.cu