Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.31 no.3 La Habana jul.-set. 2014
RESEARCH
Microbiological evaluation and pathogenicity of a liquid bioformulation of the fungus Purpureocillium sp. (strain UdeA 0109) on Meloidogyne incognita-javanica stages
Microbiological evaluation and pathogenicity of a liquid bioformulation of the fungus Purpureocillium sp. (strain UdeA 0109) on Meloidogyne incognita-javanica stages
Nadya Lorena Cardona, David Andrés Borrego, Erika Pamela Fernández, Jessika Sánchez, Valentina Cardona, Gabriel Montoya
Grupo de Biocontrol y Microbiología Ambiental, BIOMA, Facultad de Ciencias Exactas y Naturales, Instituto de Biología, Universidad de Antioquia, Calle 67 No. 53-108, Medellín, Colombia.
ABSTRACT
The BIOMA research group (Biocontrol and environmental microbiology) has an industrial liquid formulation based on Purpureocillium sp. (UdeA 0109 strain), developed with the collaboration of Laverlam S.A, a Colombian (Cali) commercial house. In the present study the researchers tested the viability and the purity at different storage temperatures as well as its biological potential both in vitro and under greenhouse conditions. The results of viability showed how the infective structures were affected neither by the evaluation time nor by the storage temperatures. Studies also showed that the purity of the bioformulation in the same conditions was over 99 %, and its pathogenicity in vitro with an LC50 of 104 conidia/mL was corroborated. The greenhouse tests showed the ability to produce damages in eggs of the Meloidogyne incognita-javanica complex, and a decreasing of the J2 stages at a concentration of 108 conidia/mL in three tests performed with a different number of applications and at different times each.
Keywords: biocontrol fungus, Purpureocillium sp., Meloidogyne incognita-javanica.
RESUMEN
El Grupo de Biocontrol y Microbiología Ambiental (BIOMA) cuenta con una formulación industrial líquida del hongo Purpureocillium sp. (cepa UdeA 0109), desarrollada de conjunto con la casa comercial Laverlam S.A. (Cali, Colombia). En la presente investigación se evaluaron la viabilidad y la pureza del producto a diferentes temperaturas de almacenamiento, así como el potencial biocontrolador in vitro y en condiciones de invernadero. Los resultados de viabilidad mostraron cómo las estructuras infectivas no se afectaron por los tiempos de evaluación ni por las temperaturas de almacenamiento. Los estudios también mostraron cómo la pureza del bioformulado bajo las mismas condiciones, se mantuvo por encima del 99 %, y además se corroboró su patogenicidad in vitro con una CL50 de 104 conidias/mL. Las evaluaciones en invernadero demostraron la propiedad de afectación de los huevos del complejo Meloidogyne incognita-javanica así como la disminución de los estadios jóvenes con una concentración de 108 conidias/mL en tres pruebas, con distintas aplicaciones y tiempos de aplicación.
Palabras clave: hongos biocontroladores, Purpureocillium sp., Meloidogyne incognita-javanica.
INTRODUCTION
One of the most damaging pests to crops and the economy is the root-knot nematode Meloidogyne spp. It infests more than 3000 species of vegetable, fruits, cereals and ornamental plants [1], impairs its roots development, reducing the plant nutrients intake and causes significant economic losses in crops. It is usually controlled by treatments with chemicals, which increase production costs due to its purchase and also lead to water and soil pollution. Current strategies to circumvent these limitations include the integrated pest management (IPM), with alternative methods to the use of chemicals, such as the use of fungi as biocontrol agents. They have been formulated in different presentations to boost its action in the field and to facilitate their use and application by the farmers.
The fungal formulations are prepared by mixing the fungi infective structures or metabolites with certain compounds, to provide stability to the active ingredient. Proper formulation should allow its prolonged storage, and a minimum loss of product qualities such as pathogenicity, viability and stability over time [2, 3]. There are several biocontrol fungi with nematicide properties that affect eggs and juvenile stages (J2) of nematodes. Among them is Purpureocillium lilacinum [4, 5].
In the group of Biocontrol and Environmental Microbiology (BIOMA), University of Antioquia, Medellin, Colombia, a biocontrol fungus against nematodes was isolated from the soil, belonging to the Purpureocillium genus which species are under study. Due to its biocontrol potential, an industrial bioformulation process was developed for a liquid presentation containing the Purpureocillium sp. strain UdeA 0109 as active ingredient. This work is part of a joint research of the BIOMA group and the Laverlam SA enterprise (Cali, Colombia). The aim was to determine the viability of the fungus Purpureocillium sp., strain UdeA 0109 on the Meloidogyne incognita-javanica complex at different times and temperatures of storage, as well as its pathogenicity in vitro and under greenhouse conditions [6, 7].
MATERIALS AND METHODS
The work was developed in two phases. First, the effect the Purpureocillium sp. strain 0109 UdeA on the Meloidogyne incognita-javanica complex was evaluated under laboratory conditions. And second, the formulation was assessed for the protection it could confer to tomato seedlings against the action of the nematode under greenhouse conditions. The project was implemented in the facilities of the BIOMA group at the Institute of Biology and the Laboratory of Biology, East Division of the University of Antioquia (Rionegro, Colombia). For the experimental process, a commercial liquid formulation was used, supplied by BIOMA laboratory to the Laverlam S.A. enterprise (Cali, Colombia), containing the UdeA 0109 strain as active ingredient. The bioreactor was scaled up in the commercial house with the information provided by the BIOMA group and signed by the two entities on the confidential agreement No. 8802-03-2010.
Laboratory assessment
Determination of germination and purity percentages
The structural behavior of the fungal formulation was determined by germination and purity conservation tests under different conditions and on different times [3]. The formulation was evaluated at four temperatures 4-8, 14, 23-25 and 30 ºC, on days 8, 15, 30 and 90. The initial concentration of the structures (conidia/mL) was determined; the formulation was dispensed into 1.5 mL tubes and stored in duplicate under the conditions described. After the evaluation times, serial dilutions were made and 5-µL aliquots from the 1/1000 dilution were seeded in Petri dishes containing water-agar. Two Petri dishes were used for each tube and incubated for 24 hours at 23-25 °C. Then, fragments of agar inoculated with the formulation were stained with lactophenol blue solution and observed under a light microscope with a 40× magnification. Fungi germination was estimated as the ratio of germinated and nongerminated conidia, with germinated ones established as those which germ tube length exceeded twice the conidia diameter.
The purity percentage was evaluated on the same times and under the same conditions as for the germination tests. One hundred microliters of the 10-3 and 10-4 conidia/mL dilutions were plated in potato dextrose agar (PDA) medium (Merck®) in duplicate. After one week of incubation, the purity percentage (% P) was calculated as a ratio of colony forming units (c.f.u.): % P = c.f.u. of the evaluated fungus / total c.f.u. × 100.
The experimental unit consisted on tubes containing the formulation, each with five replicates per experimental condition. A nonformulated fungal suspension in distilled water was used as control and subjected to the same test conditions than the formulation. Differences between the treatments were established by an ANOVA test (p = 0.05) followed by the Tukey’s multiple comparison test. In those cases where the data did not meet statistical assumptions, sine and arcsine transformations were made and the Wilcoxon test was applied. Because of there was no variability among treatments under the conditions evaluated, in purity tests only descriptive analyses were made.
Evaluation of pathogenicity in vitro on Meloidogyne spp. eggs complex
Pathogenicity tests were done with the formulation stored at the average room temperature (23-25 ºC) for two months in the BIOMA laboratory at the University of Antioquia.
The eggs of the Meloidogyne spp. complex were obtained from nematodes multiplied under greenhouse conditions. They were extracted by the NaOCl technique (5 %) [6], further washed with 5 % oxitetracycline for 10 min, rinsed with sterile running water and stored at 4-8 °C until testing. Eggs suspensions were prepared to 200 eggs/mL for the test.
Aliquots of 1 mL of eggs and 4 mL of fungus at the concentrations to be evaluated were taken, which were collected in Petri dishes of 5 cm diameter, incubated at room temperature (25-28 °C) and evaluated after 5 days. Eggs suspended in sterile distilled water and in fungus without bioformulation were used as controls. The experimental unit was the Petri dish, and five treatments were evaluated (102, 103, 105, 107 and 108 conidias/mL) of the bioformulation stored at room temperature (23-25 °C) after 90 days, each with five repetitions. The evaluated variable was the percentage of morphological variation of eggs per experimental unit. A completely randomized design was used. Significant differences were determined by ANOVA (p = 0.05), followed by the Tukey’s multiple comparison test. Affected eggs were stained with lactophenol blue and photographed under the microscope (Olympus Model BX 60-F5), with a 40× magnification.
Evaluation under greenhouse conditions
Determination of fungal persistence in soil
The persistence over time of the Purpureocillium sp. strain UdeA 0109 strain on the soil was investigated to establish the optimal application frequency of the fungal bioformulation. Nine 300-mL plastic cups were used, containing 114.5 g of sterile dry soil each and distributed at three cups per concentration tested (103, 105 and 108 conidias/mL).
Samples of 5 g of soil were taken from each cup at 5-days intervals on days 5, 10, 15 and 20 days after incubation. On each time, serial dilutions were made, and 100 μL of each 10-4 conidias/mL dilutions were plated on Petri dishes containing acidified PDA medium, in duplicates, and further incubated for 7 days at 25-28 °C to determine the number of c.f.u./g. The results obtained were subjected to a descriptive analysis and the application time of the fungus was established for the pathogenicity test under greenhouse conditions.
Moisture conditions, required for both the establishment of the fungus and the survival of nematode (70 %), were established by calculating the maximum moisture retention capacity of the soil [8].
Determination of the optimal concentration of the formulation to reduce the number of juvenile stages of Meloidogyne spp. on greenhouses
The test was performed in the greenhouse at the University of Antioquia, Medellin, Colombia, at an average temperature of 26 ºC. The soil was sterilized by autoclaving and subsequently dried inside the greenhouse to humidity levels below 50 %. For the first inoculation of the formulation provided by the commercial house, 840 mL of the spore solution was applied to 16.8 kg of soil, equivalent to the substrate for cultivation of 21 plants, in a 100-L plastic container to final moisture of 70 %. The initial inoculum of treatments was further applied, manually mixed and incubated for 24 h until fungus establishment. Subsequently, 30 × 25 cm plastic bags were filled with 800 g of the fungus-inoculated soil, incubated for another 24 h, and tomato seedlings were planted on them prior to its inoculation around the stem with 5 mL containing 3000 eggs of Meloidogyne spp. per plant.
Extraction of eggs and juvenile stages of Meloidogyne spp. for density estimations
Juvenile stage nematodes were extracted by the pie-pan method [9], by using sieves 10 cm high and 17 cm in diameter (Kleenex®), and a plastic mesh of 0.4 cm pore size. Soil was homogenized and 100 g taken, which were added on each sieves containing double tissue paper. The sieves were placed on disposable plates containing running water, assuring the soil remained wet. After 48 h, juvenile stage nematodes were recovered and concentrated in 100 mL for subsequent counting with the aid of an inverted optical microscope (Nikon Eclipse TS 100). Nematode eggs were extracted from the roots of each plant. Roots were finely chopped and placed in glass vials containing 50 mL of 3 % NaOCl. The suspensions were stirred at 180 rpm for 15 min, and filtered through a monofilament mesh fabric and then sieved by a 0.125 and 0.025 mm sieve set. Then, the eggs collected were washed thoroughly with water to remove the excess hypochlorite and counted as previously described for juvenile stages.
The assay consisted of three tests, each with six treatments corresponding to the concentrations of the formulation (102, 103, 105, 107 and 108 conidia/mL and the control group). Control plants were grown in non-inoculated soil and further inoculated with nematode eggs. Each treatment had seven replicates, for up to 42 plants per test. After the initial inoculum, applications were made every 15 days with 40 mL at the given concentration. Thereby, the first test included three applications, with the study of J2 nematode populations and eggs at 45 days (1.5 months); the second, five applications and evaluation after 75 days (2.5 months); and the third, seven applications of the formulation and evaluation after 105 days (3.5 months). Treatments were arranged inside the greenhouse. Each test was separated with a random distribution of plants. During the study, the moisture was adjusted to 70 % every 48 h.
The experimental unit was the plant treated with the bioformulation of Purpureocillium sp. under evaluation and the eggs solution of Meloidogyne spp. Assessed variables were the expressed as the juvenile stages/100 g of soil and eggs/100 g of roots. The differences between treatments and their controls were determined by the Wilcoxon signed-rank non-parametric test (p = 0.05), using the statistic package R (R Project, version 2.12.2).
RESULTS AND DISCUSION
Laboratory evaluation
Germination and purity at different temperatures and times of storage
The formulation concentration corresponded to 3.6 × 108 conidia/mL. No significant differences were found between controls and treatments (p ≥ 0.05) on days 0, 8 and 15, according to the Wilcoxon test. However, on day 8 significant differences between treatments were observed, as determined by the Kruskal-Wallis test. The best germination percentages (90 %) corresponded to room temperature (23-25 °C) and to greenhouse temperature (4-8 °C) (Figure 1A). There were differences between treatments at room temperature (75 % of germination) and at the temperature of Eastern Antioquia (80 % of germination) on day 15 (Figure 1B). But on 30, differences between treatments were unapparent and germination was kept between 75 and 80 % (Figure 1C). At the final determination (day 90), germination was kept between 85 and 90 % at all temperatures, a high standard for a commercial formulation (Figure 1D). This has been reported for other fungi, such as Beauveria bassiana in an oil surfactant formulation which showed increased tolerance at high temperatures, preserving its virulence and maintaining conidia viability over time [9].
It is important to add that the formulation was presented as a homogeneous suspension without mycelial growth on the surface during the entire study, which was observed in the controls. Similarly, several authors have described that there are formulation processes which do not affect the spores, and, to the contrary, improve the stability and useful lifespan during storage of these structures [3, 10-13].
Purity ranged 99-100 % for all the treatments and conditions, above the 90 % recommended by quality control standards for entomopathogenic fungi [3, 10]. It indicated an adequate formulation quality management by the trading house (Laverlam).
According to these results, the liquid commercial formulation displayed no adverse effect on germination or in purity for the structures of the UdeA 0109 strain at times and temperatures evaluated.
In vitro pathogenicity testing
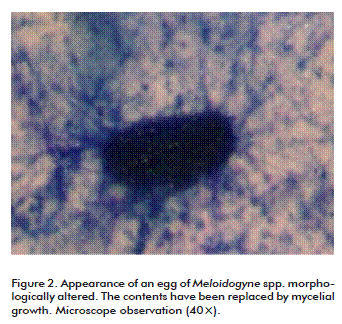
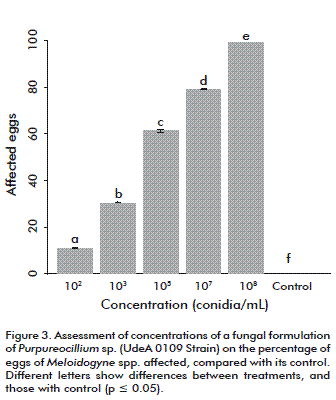
The analyses showed that the 104 conidia/mL concentration may affect 50 % of nematode population, according to the mean lethal concentration (LC50) after 5 days. Hence, it was decided to assess lower and higher concentrations under greenhouse conditions. The LC50 exceeds that of comercial nematicides bioformulations of P. lilacinum and Prochonia chlamydospora used in vitro, which achieve nematicidal effects at concentrations of 1.03 × 108 spores/mL on developmental stages of Radopholus similis [14]. This implies the possibility that the product could be used at lower concentrations in the field, an aspect that should be further addressed. The dosage effect was evaluated by determining the morphological alteration of nematode eggs. Such alteration was attained in 10 % of the eggs at a concentration of 102 conidia/mL and up to 99 % at 108 conidia/mL (Figure 2 and Figure 3). Moreover, the formulation was able to affect the eggs after two months of storage at room temperature, although qualitative evaluation continued and showed viability after seventh months (data not shown), another aspect recommended for this type of formulations.
Evaluation under greenhouse conditions
Determination of fungal persistence in soil
At start, the inoculation time, conidia concentration decreased from initial levels applied for all the three concentrations evaluated. Fungal c.f.u. counts were found increased on day 5, remained steady on days 10 and 15, and further declined on day 20. Based on these results, the time interval to apply the bioformulation was set to 15 days, to keep constant the spore density in soil for an effective long term control of Meloidogyne spp. and also to reduce the amount of bioformulation used. Other groups [15] found that P. lilacinum u.f.c. counts decreased from those added at the start of the experiment, as well as other teams have reported that c.f.u. decrease from two to three weeks after the initial application [16, 17].
J2 juvenile stage population assessment
The J2 juvenile stage nematode populations in 100 g of soil were significantly reduced (p ≤ 0.05) by three applications of the bioformulation at 105, 107 and 108 conidia/mL, compared to the control and the other treatments (Figure 3). The highest numbers of J2 individuals were found at 102 and 103 conidia/mL (79 and 57 nematodes, respectively), with as few as 14 nematodes with the 102 concentration (Figure 4A).
More than 90 % of the eggs were affected at any concentration, with significant differences for all the treatments compared to the control (p ≤ 0.05) (Figure 4D).
After five applications, there were no significant decrease in nematode populations for the 102, 103, 105 and 107 conidia/mL concentrations compared to the control, with Meloidogyne spp. J2 individuals counts ranging 164-329 nematodes/100 g of soil. There were only 14 nematodes for the 108 concentration (Figure 4B). Otherwise, all the concentrations significantly affected the eggs (p ≤ 0.05), from 50 to 90 %. The highest effect was found at the 108 concentration (Figure 4E), regardless the lack of differences with results achieved at 107 conidia/mL. Increased J2 nematode populations were found for treatments at the lowest concentrations (102 and 103) and also the control, with five applications of the bioformulation. This result could derive from successful plant root colonization from the start of the experiment by those populations which ended their reproductive cycle, in spite of being the second evaluation. In fact, root knots were present in the analyzed material. In previous studies, we showed that the UdeA 0109 strain did not display an endophytic behavior, only attacking exposed nematode populations. In this sense, it was reported the high susceptibility of tomato against Meloidogyne spp. and, depending on the variety, gall formation could appear, further increasing eggs production, some of them occluded in inner tissues what probably protect them from fungal infection [18]. All these makes essential to determine the preventive effect of the product, which should have to be studied in field experiments, since some authors have reported that other P. lilacinum strains have to be applied before trasplantation to reduce the inoculum of nematodes in soil, their reproduction and the associated root damage [16, 19].
Another aspect that could have influenced on the increase of J2 nematode population was the procedure used to inoculate the bioformulation in soil. According to the methodology used, the fungus was directly mixed with the soil during the first inoculation, while the rest of the inoculations were done by aggregating the strain on the surface around the seedling stem, without mixing it with soil. Other authors found that the root system and the depth of fungus inoculation could be relevant for its biocontrol properties on Meloidogyne sp. populations [15]. These aspects require careful assessment in future studies.
In the treatment with seven inoculations of the bioformulation, significant differences were found for J2 nematodes at 108 conidia/mL compared to the control (p ≤ 0.05) (Figure 4C), account for 21 J2 nematodes per 100 g of soil. The rest of concentrations did not show differences compared to the control. Conversely, there were significant differences in eggs counts (p ≤ 0.05) for all the concentrations tested in respect to the control, with a 100 % effect on eggs at 107 and 108 conidia/mL. Once again, the 108 conidia/mL concentration was considered optimal since it kept the lowest populations of J2 nematodes. Noteworthy, some fungi strains could vary, requiring concentrations higher than 1 × 107 spores/mL to establish effective infection, as described [20]. It has also been reported that a P. lilacinum concentration of 106 or higher has to be maintained in soil [19, 21], a result confirmed in the present study with the 107 and 108 as the best effect concentrations.
CONCLUSIONS
The microbiological quality of a liquid formulation of the Purpureocillium sp. UdeA0109 strain and its pathogenicity on developmental stages of the Meloidogyne incognita-javanica complex were verified. Further research should elucidate if the 107 conidia/mL concentration registered as the most effective in this study exerts better control on nematode populations than other biological nematicides available in the market with effective concentrations ranging 108-1012 conidia/mL [22]. Noteworthy, the results provide information on the application frequency of the bioformulation to decrease nematode eggs and J2 juvenile stage populations under greenhouse conditions, useful to validate the results under field conditions.
ACKNOWLEDGEMENTS
The authors thank to the members of BIOMA group for their contributions to the development of the research. This work was funded by the Committee for the Development of Research (CODI) of the University of Antioquia (Medellin, Colombia) and the company Laverlam SA (Cali, Colombia) under the Code E01568.
REFERENCES
1. Abad P, Favery B, Rosso MN, Castagnone-Sereno P. Root-knot nematode parasitism and host response: molecular basis of a sophisticated interaction. Mol Plant Pathol. 2003;4(4):217-24.
2. Batista FA, Alves SB, Alves LFA, Pereira RM, Augusto NT. Formulção de entomopatogenos. In: Controle Microbiano de Insetos. 2 ed. São Paulo: FEALQ; 1998. p. 917-66.
3. Vélez A, Posada F, Marín P, González G, Osorio VE, Bustillo P. Técnicas para el control de calidad de formulaciones de hongos entomopatógenos. Bol Téc Cenicafé. 1997;17:1-37.
4. Luangsa-Ard J, Houbraken J, van Doorn T, Hong SB, Borman AM, Hywel-Jones NL, et al. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol Lett. 2011;321(2):141-9.
5. Hewlett TE, Dickson DW, Mitchell DJ, Kannwischer-Mitchell ME. Evaluation of Paecilomyces lilacinus as a biocontrol agent of Meloidogyne javanica on Tobacco. J Nematol. 1988;20(4):578-84.
6. Sánchez JY. Estudio de viabilidad de las estructuras del hongo Purpureocillium sp. cepa UdeA0109 formulado en un ingrediente inerte bajo diferentes condiciones de tiempo y temperatura. Trabajo de grado para optar por el Título de Biólogo. Universidad de Antioquia. Facultad de Ciencias Exactas y Naturales. Seccional oriente - Carmen de Viboral. Grupo BIOMA. Universidad de Antioquia; 2013.
7. Cardona HV, Montoya TGJ. Evaluación de la patogenicidad del bioformulado de esporas de Purpureocillium spp. (Cepa UdeA 0109) sobre poblaciones de Meloidogyne spp. en plantas de tomate (Solanum lycopersicum) bajo condiciones de invernadero. Trabajo de pregrado para optar por el título de Biólogo. Medellín (Colombia). Grupo BIOMA. Universidad de Antioquia; 2013.
8. Margesin R, Schinner F. Manual for soil analysis - Monitoring and assessing soil bioremediation. Soil Biology Vol. 5. Berlin: Springer; 2005.
9. Coyne DL, Nicol JM, Claudius-Cole B. Practical plant nematology: a field and laboratory guide. SP-IPM Secretariat. Cotonou: International Institute of Tropical Agriculture (IITA); 2007.
10. Bastidas A, Velásquez SE, Marín P, Benavides P, Bustillo AE, Orozco FJ. Evaluación de preformulados de Beauveria bassiana (bálsamo) Vuillemin, para el control de la broca del café. Agron. 2009;17(1):44-61.
11. Hong TD, Edgington S, Ellis RH, de Muro MA, Moore D. Saturated salt solutions for humidity control and the survival of dry powder and oil formulations of Beauveria bassiana conidia. J Invertebr Pathol. 2005;89(2):136-43.
12. Gato CY. Métodos de conservación y formulación de Trichoderma harzianum rifai. Fitosanidad. 2010;14(3):189-95.
13. Urtuvia UH, France A. Formulaciones de hongos entomopatógenos para control de plagas. Inia Tierra Adentro. 2007;(nov.-dic.):46-9.
14. Alzate D, Guzmán OA, Leguizamón Caycedo J. Efecto in vitro de Purpureocillium lilacinum (Thom) Luangsa, Ard et al. y Pochonia chlamydosporia (Goddard) Zare y Gams sobre el nematodo barrenador Radopholus similis (Cobb) Thorne. Agron. 2012;20(2):25-36.
15. Anastasiadis IA, Giannakou IO, Prophetou-Athanasiadou DA, Gowen SR. The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop Protect. 2008;27:352-61.
16. Kiewnick S, Mendoza A, Sikora RA. Efficacy of Paecilomyces lilacinus strain 251 for biological control of the burrowing nematode Radopholus similis [Abstract]. J Nematol. 2004;36:326-7.
17. Kiewnick S, Sikora RA. Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol Control. 2006;38(2):179-87.
18. López-Llorca LV, Jansson HB. Biodiversidad del suelo: control biológico de nemátodos fitopatógenos por hongos nematófagos. Cuadernos de Biodiversidad. 2001;(6):12-5.
19. Stirling GR. Biological control of plant parasitic nematodes: soil ecosystem management in sustainable agriculture. 2ed. Wallingford: CABI; 2014.
20. Sahebani N, Hadavi N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol Biochem. 2008;40(8):2016-20.
21. Mendoza AR, Sikora RA, Kiewnick S. Influencia de la dosis y el tiempo de aplicación de Paecilomyces lilacinus cepa 251 sobre el control biológico del nematodo barrenador Radopholus similis en banano. Nematropica. 2007;37(2):203-13.
22. Corporación Colombiana de Investigación agropecuaria CORPOICA. Listado de empresas de bioinsumos registradas. 2013 [cited 2013 Oct 2]. Available from: http://www.ica.gov.co/Areas/Agricola/Servicios/Fertilizantes-y-Bio-insumos-Agricolas/Listado-de-Bioinsumos/2009/EMPRESAS-REGISTRADAS-BIOINSUMOS-JULIO-8-DE-2008.aspx.
Received in November 2013.
Accepted in October 2014.
Nadya Lorena Cardona. Grupo de Biocontrol y Microbiología Ambiental, BIOMA, Facultad de Ciencias Exactas y Naturales, Instituto de Biología, Universidad de Antioquia, Calle 67 No. 53-108, Medellín, Colombia. E-mail: nadya.cardona@udea.edu.co; nadyaloren@gmail.com.