Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Plantas Medicinales
versión On-line ISSN 1028-4796
Rev Cubana Plant Med vol.16 no.4 Ciudad de la Habana oct.-dic. 2011
ARTÍCULO ORIGINAL
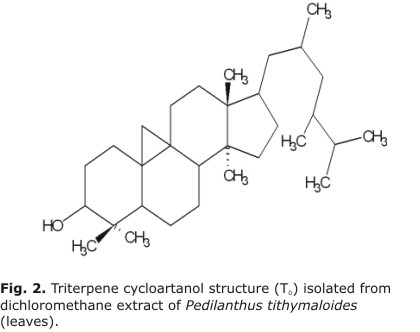
Characterization of cycloartanol triterpene isolated from dichloromethane extract of Pedilanthus tithymaloides
Caracterización del triterpeno cicloartanol aislado del extracto de dicloromentano de Pedilanthus tithymaloides
MSc. Hirán Cabrera Suárez,I Dr. C. Armando Cuellar Cuellar,II MSc. Roberto Carlo PitaIII
I Central Laboratory of Pharmacology (LCF), Medical Sciences Faculty "Dr Salvador Allende". La Habana, Cuba.
II Pharmacy and Food Institute, Havana University. La Habana, Cuba.
III Synthetic Antigen Center, Havana University. La Habana, Cuba.
ABSTRACT
Introduction: Pedilanthus tithymaloides is known as ítamo real and it has been previously reported as an alternative to treatment of oral cavity virus infections.
Objective: to explain procedures to characterize one of the major compounds isolated from dichloromethane extracts of Pedilanthus tithymaloides (L.) Poit (leaves).
Methods: it was obtained cycloartanol from dichloromethane extract modifying polarity of the solvents with methanol and by increasing polarity to extract, they were used chromatography technique and spectroscopic of nuclear magnetic resonance H+ and 13C to characterize this compounds.
Results: phytochemical studies showed quality parameters that were similar to average limits in the use of majority of medicinal plants except total ashes assay (13 %), qualitative determination assay suggest presence of reducers compounds, quinone, phenols and/or tannins, triterpene and/or steroids. And they were compared spectrum carbon 13 (13C) of the substance obtained from extract of this plant with other reported in literature to cycloartanol and found coincidence in majority of carbons compared.
Conclusions: cycloartanol triterpene was one of main compounds of dichloromethane extract of Pedilanthus tithymaloides (L) Poit leaves.
Key words: Pedilanthus tithymaloides, Cycloartanol, Triterpene, Itamo real, Phytochemistry.
RESUMEN
Introducción: Pedilanthus tithymaloides es conocido como ítamo real y se han publicado algunas de sus propiedades, entre ellas, el uso en estomatología para el tratamiento de infecciones bucales.
Objetivo: explicar los procedimientos empleados para caracterizar uno de los compuestos mayoritarios aislado del extracto de diclorometano a partir de las hojas de Pedilanthus tithymaloides (L.) Poit.
Métodos: el cicloartanol se obtuvo mediante la modificación de la polaridad del disolvente orgánico, con el empleo de metanol e incrementándose la polaridad y como consecuencia la aparición de un precipitado. Se utilizó la técnica de cromatografía de placa delgada, la cromatografía de columna, así como espectroscopia de resonancia magnética nuclear protónica y de carbono 13.
Resultados: el estudio fitoquímico mostró parámetros de calidad acorde a lo que se sugiere para el uso de plantas medicinales, excepto las cenizas totales que presentaron 13 %; se comparó el espectro de carbono13 practicado al compuesto obtenido con otros reportes en la literatura y se encontró coincidencia con lo que se describe para el triterpeno cicloartanol.
Conclusiones: cicloartanol es uno de los compuestos mayoritario presente en el extracto de dicloromentano obtenido a partir de hojas de Pedilanthus tithymaloides
Palabras clave: cicloartanol, Pedilanthus tithymaloides, ítamo real, triterpenos, fitoquímica.
INTRODUCTION
Secondary metabolites obtained from natural sources are very important to treat several human diseases.
Pedilanthus tithymaloides (L.) Poit. (Euphorbiaceae) has been reported to have a wide range of properties, namely as emetic, antiinflammatory, antibody, antiseptic, antitumoral, and abortive effects.1-4
Some preclinical studies to evaluate the neurosedative effect of dichloromethane extract obtained from Pedilanthus tithymaloides (L.) Poit. leaves, showed results of several tests that indicate a depressing effect on the central nervous system.5
There were found some reports about neurosedative effects related with triterpene compounds6-9 and phytochemical studies have reported triterpenes in organic extract of P. tithymaloides.10
Today, there are few reports about the chemical composition of this plant; therefore, this review was aimed at explaining procedures to characterize one of majority compounds isolated from dichloromethane extracts of Pedilanthus tithymaloides (L) Poit leaves.
METHODS
The plant leaves were collected in the orchard of medicinal plants of Pharmacy and Food Institute of Havana University. It was authenticated by Ph.D. Mónica Sakaraqui of University of Maringa, Paraná, Brazil, with number of herbarium 6104
Extracts preparation
It was carried out by continuous extraction (Soxhlet) with 500 mL of dichloromethane and 100 g of the vegetable material dried in the stove and fractional previously (5 mm), it was processed during 10 hours.
Obtaining cycloartanol triterpene
Each gram of extract dried was dissolved in 5 mL of chloroform and 20 mL of methanol, this process allowed obtaining a greenish-yellow solid precipitate; they were recovered and separated by filtration from an oily consistency mother solution (T0S). The greenish yellow solid was processed to purification by successive washing with acetone and a white solid (T0) was obtained, and it was characterized by NMR H+ and NMR 13C.
Chromatographic studies
The qualitative analysis based on chromatography it was carried out through thin plates of silica gel (G-60, F-254) to thin layer chromatography (TLC). Some 50 % sulfuric acid and heat or ultraviolet light (UV) 254 nm werw used to visualize spots.
Spectral analysis
The spectrum of magnetic resonance of H+ and 13C were carried out in a Brucker ac. 250 MHz equipment with chloroform deuterium and tetramethilsilane as internal reference.
RESULTS
The dichloromethane extract presents an intense green color. It was a 2 % of weight, respect of vegetable material (leaves).
Dried process in stove lasted 3 days and 83 % of water was extracted from it. Relative humidity was 12 %. The total ashes were 13,02 %, while the value of ashes was 11,83 % this agreed with alkaline metals. Qualitative determination suggested to presence of quinone, reducing compounds, phenol and/or tannin, triterpene and/or steroids.
Spectroscopic datas
NMR H+ (CDCl3, 250 MHz). 4,2 ppm (OH), 3,3 ppm (C 3), 2,3 ppm, 2,1 ppm, 1,3 ppm. 0,9 ppm
The most important signals were located between 0,5 ppm and 2,4 ppm in correspondence with nucleus of triterpene, spectrum showed others signals next to 4 ppm in correspondence with OH to C 3 and 3,3 related to H of C 3. All of this data was not enough to characterized this molecule, so it was necessary to analyse spectrum of NMR 13C.
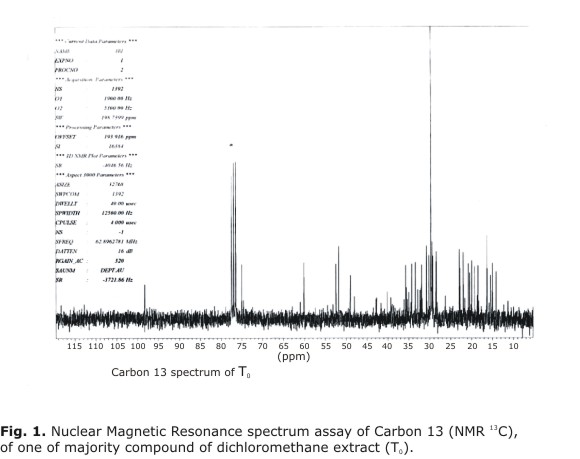
NMR 13C (T0) (CDCl3, 250 MHz): 14.79 ppm (C-30), 15.69 ppm (C-31),16.07 ppm (C-27),16.95 ppm (C-18), 17.02 ppm (C-21),19.89 ppm (C-21), 19.89 ppm (C-9), 20.62 ppm (C-26), 21.14 ppm (C-6), 21.39 ppm (C-11), 23.57 ppm (C 29), 26.89 ppm (C 16), 29.07 ppm (C 7), 29.90 ppm (C 19), 30.05 ppm (C 2), 30.86 ppm (C 25), 31.45 ppm (C 23), 32.61 ppm (C 1), 32.78 ppm (C 15), 34.10 ppm (C 22), 34.98 ppm (12), 36.26 ppm (10), 36.28 ppm (20), 39.96 ppm (C 24), 40.84 ppm (C 13), 43.54 ppm (C 8), 48.75 ppm (C14), 49.70 ppm (C 5), 52.48 ppm (C 17), 75.77 ppm (C 3) (Fig. 1).
Carbon 13 spectrum of T0 was compared with others reported in literature and they were found coincidence with C13 spectrum of cycloartanol.
NMR 13C cycloartanol (CDCl3, 250 MHz): 14 ppm (C-30), 15.5 ppm (C-31),17.6 ppm (C-27),17.9 ppm (C-18), 18.4 ppm (C-21), 20 ppm (C-21),20.05 ppm (C-9),21.1 ppm (C-26),21.1 ppm (C-6), 21.4 ppm (C-11), 25.24 ppm (C 29), 26.5 ppm (C 16), 28.8 ppm (C 7), 29.6 ppm (C 19), 30.33 ppm (C 2), 30.9 ppm (C 25), 31.4 ppm (C 23), 31.9 ppm (C 1), 32.9 ppm (C 15), 33.9 ppm (C 22), 35.5 ppm (12), 36.0 ppm (10), 36.5 ppm (20), 39.0 ppm (C 24), 40.1 ppm (C 13), 43.8 ppm (C 8), 48.7 ppm (C14), 50.1 ppm (C 5), 52.1 ppm (C 17), 78.5 ppm (C 3).
Only carbons 18, 21, 27 have different 1 ppm but it is acceptable according to reports of Wehrli and Varian in 1978 (Fig. 2).
DISCUSSION
Triterpenes are widely distributed in the plants and animals and they can be classified as not cyclic, tetracyclic and pentacyclic, these compounds can be extract from plant using organic solvents.11
There are different methods described by literature to isolate triterpenes from extracts, one of them is modifying polarity of the solvents, other methods use preparative chromatography techniques by means of thin layer (TLC) and preparative chromatography techniques by means of column among others techniques.11-13
Nuclear magnetic resonance (NMR) is based in magnetic properties of atomic nucleus, transition produced by an external magnetic field and it can be detected as a weak signal of absorption.14,15
Nuclear magnetic resonance (NMR) is an indispensable analytical technique to identify drug metabolites, to determine degradation products of a drug, and to elucidate impurities in a drug substance, although it is generally considered an expensive and insensitive technique and that is why it is more advisable to use, high performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS), or liquid chromatography-mass spectrometry (LC-MS) methods, to monitor the clearance of small-molecule impurities.14,15
Since the discovery more than 50 years ago, nuclear magnetic resonance (NMR) spectroscopy has been an important analytical method for chemists. The introduction of FT (fourier transform) NMR techniques made it even more powerful and widely used. NMR is undoubtedly one of the most valuable tools in the discovery stage of pharmaceutical product development.14,15
Each spectrum are in correspondence with an specific molecule, so it is acceptable to compare spectrum and relation their common characteristics in order to try to know compounds isolated from extracts or synthesized.14,15
Therefore they were reporting to triterpene cycloartanol as one of major compound isolated from dichloromethane extract of P. tithymaloides leaves.
REFERENCES
1. Cuba. Ministerio de Salud Pública. Plantas Medicinales (Fitomed III). La Habana: Editorial Ciencias Médicas; 1994. p. 34-5.
2. Roig JT. Diccionario botánico de nombres vulgares cubanos. La Habana: Editorial Científico-Técnica; 1974.
3. Beltrán A, Cuellar A. Evaluación antitumoral del látex de la planta ítamo real [Tesis de Diploma]. IFAL: Universidad de La Habana; 1999.
4. Roig JT. Diccionario botánico de nombres vulgares cubanos. 3ra ed. La Habana; 1962. p. 1092-3.
5. Cabrera Suárez H, Núñez Figueredo. Efecto sedante del triterpeno cicloartanol obtenido de las hojas de Pedilanthus tithymaloides (L.) Poit. Rev Cubana Plant Med [online]. 2010[citado 21 May 2011];15(3):96-104 . Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962010000300001&lng=es&nrm=iso ISSN 1028-4796.
6. Rojas G, Aranda E, Navarro V, Zamilpa A, Tortoriello J. In vitro propagation of Galphimia glauca and content of the sedative compound galphimine-B in wild and micropropagated plants. Planta Med. 2005;71(11):1076-8.
7. Amos S, Orisadipe A, Binda L, Emeje M, Adesomoju A, Okogun J, et al. Behavioural effects in rodents of methyl angolensate: a triterpenoid isolated from Entandrophragma angolense. Pharmacol Toxicol. 2002;91(2):71-6.
8. Osuna L, Pereda-Miranda R, Tortoriello J, Villarreal ML. Production of the sedative triterpene galphimine B in Galphimia glauca tissue culture. Planta Med. 1999;65(2):149-52.
9. Tortoriello J, Ortega A. Sedative effect of galphimine B, a nor-seco-triterpenoid from Galphimia glauca. Planta Med. 1993;59(5):398-400.
10. Sánchez Govín E, Pérez Lamas AM, Chávez Figueredo D, Hechevarría Sosa I. Caracterización farmacognóstica de Pedilanthus tithymaloides L. Poit. Rev Cubana Plant Med [serie en Internet]. 2005 Abr [citado 02 Oct 2011];10(1). Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962005000100004&lng=es
11. Cuellar A, Miranda M. Farmacognosia y productos naturales. La Habana: Editorial Félix Varela; 2001. p. 207-13.
12. Satyajit D. Sarker, Zahid Latif, Alexander I. Gray Natural Products Isolation 2nd ed. New Jersey: Human Press Inc.; 2006. ISBN 1-59259-955-9
13. Bhakuni DS, Rawat DS. Bioactive Marine Natural Products. New York: Ed. Springer; 2005. p. 64-13. ISBN 1-4020-3484-9 (e-book).
14. Wehr T, Rodriguez Diaz R, Tuck S. Analytical techniques for biopharmaceutical development. New York: Ed. Marcel Dekker; 2005.
15. Breitmaier E. Structure Elucidation by NMR in Organic Chemistry: A Practical Guide. 3rd ed. England: John Wiley & Sons, Ltd.; 2002.
Recibido: 5 de julio de 2011.
Aprobado: 26 de septiembre de 2011.
Hirán Cabrera Suárez. Central Laboratory of Pharmacology (LCF), Medical Sciences Faculty "Dr Salvador Allende". Carvajal s/n and Agua Dulce, Cerro, La Habana, Cuba. E-mail: irancs@infomed.sld.cu