Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Ciencias Médicas de Pinar del Río
versión On-line ISSN 1561-3194
Rev Ciencias Médicas vol.27 no.1 Pinar del Río ene.-feb. 2023 Epub 01-Ene-2023
Articles
Prototype application on Hemovigilance System in the province of Pinar del Rio
1Universidad de Ciencias Médicas de Pinar del Río. Banco Provincial de Sangre. Pinar del Rio, Cuba
2Universidad de Ciencias Médicas de Granma. Dirección Provincial de Salud. Granma, Cuba.
Introduction:
the process of information management covers from the promotion of donation, donor selection, blood collection, donation complications, processing and analysis of blood components, transfusion and adverse and unexpected effects that the donor and recipient may present. the study is carried out because it is necessary to improve information management in order to optimize processes, workflow, analysis, registration and control. Therefore, it was decided to develop a prototype of its own application that complies with the necessary parameters for the work in the Hemovigilance System in Pinar del Rio Province.
Objective:
to elaborate a prototype of a computer application for the management of the hemovigilance system in Pinar del Rio province.
Methods:
a research study was carried out with the objective of developing the information architecture on which the hemovigilance system management process is based, applying the theoretical and empirical historical-logical, inductive-deductive, conversational and documentary analysis methods. Using the XP development methodology.
Results:
the system allows recording, visualizing and controlling the development of the information generated by the Hemovigilance System in Pinar del Río.
Conclusions:
It was obtained the design of a prototype of a computer application for the management of the hemovigilance system in Pinar del Río province that will allow the timely control for decision making at each stage of the process.
Key words: BLOOD SAFETY; GIFT GIVING; TRANSFUSION
INTRODUCTION
Blood is essential for life, since only the human being is capable of producing it, but its duration is short. Human blood is so complex that no one has been able to reproduce it artificially. Thousands of people need this vital liquid, due to accidents, surgeries, violence, birth complications, platelet deficiency, hemophilia, leukemia, cancer, among others.
The first real advances in the use of blood were made in 1901 with the discovery of the existence of blood groups (types A, B and O) and in 1940 with the discovery of the Rh factor by the scientist and later Nobel Prize in Medicine Karl Landsteiner.
Among the ideas and contributions made after World War II, it is worth mentioning Carl Walter and W.P. Murphy in 1950, when they introduced the plastic bag which left behind the fragile glass bottle as a container.1
In the second half of the 20th century, blood component therapy made it possible to match individual blood components to the needs of patients. Voluntary blood donors came to play an important role as collaborators with health care professionals around the world.
In the following years, the amounts of sodium citrate were adjusted. It was found that cold preserved the blood.1
In 1930 the first blood banks began to operate, both in Europe and North America. The need for new research on blood transfusion increases, at the same time as the world is revolutionized and new technologies emerge.2
The first advances towards achieving a systematic hemovigilance process date back to 1995 when the European Council published its resolution of June 2 and work began on blood safety and blood self-sufficiency in the community with the aim of improving public confidence in the safety of the blood supply as a consequence, aspects of hemovigilance, mainly the occurrence of adverse events, became legally regulated.3
At present, blood donation in Cuba is an altruistic act, voluntary and free of reimbursement, which implies with a higher degree of probability the obtaining of safe blood. Many developing countries, such as Peru, still rely on relatively unsafe donations.
In order to obtain with greater probability a high number of safe donations, we consider that research and investment focused on improvements in the processes of selection and identification of potential blood donors should be continuous, and supported by the current guidelines of the National Program of Hemotherapy and Blood Banks. However, we suggest the constant implementation of measures in the current programs that allow the education of the population regarding the importance of voluntary blood donation.4
Hemovigilance is defined as the set of procedures that establishes the need to have an organized control over the adverse effects or reactions that manifest themselves in blood donors and recipients, which may appear throughout the transfusion chain, as well as their epidemiological follow-up. It covers from the collection of whole blood or the component by apheresis, to the follow-up of the recipients, in order to prevent and treat the appearance or recurrence of adverse events.4
For the analysis and correction of the causes and consequences of adverse reactions, a working methodology should be established in which all personnel involved in the donation, production and transfusion processes participate. Hence the importance of hemovigilance in the donor-recipient chain.5
The organization of voluntary blood donation in Cuba began in 1962 and contributions have increased systematically.
Since the 80's of the last century, work has been done on the consistent and progressive application of quality standards to guarantee the safety of blood components 7), and the first experience of a hemovigilance program was carried out in the province of Matanzas in 2003, the results of which were published in 2009.7
The Cuban Blood Program, which has 48 banks and 164 transfusion services, guarantees the production of blood products, which have contributed to raise health indicators in the country and in 22 other nations.
Thus, hemovigilance emerges as a system for the detection, registration, analysis and control of information starting with the promotion of donation and selection of donors, blood collection, donation complications, processing and analysis of blood components and finally, transfusion and the adverse and unexpected effects that the recipient may present.
This information ensures that a continuous quality control of the transfusion chain is established, a fact that brings indisputable benefits to both transfused patients and blood donors.
Many of the activities related to data collection are carried out manually by the personnel working in the blood banks and transfusion services. These activities, which must be carried out daily, imply a waste of time on the part of the health personnel, as well as the possible introduction of human errors and the loss of timely information aimed at hemovigilance from the donor to the recipient of the components or blood derivatives, as well as the control of the material resources used in the process. At present, in the province of Pinar del Río it represents a problem to be solved.
In order to provide a solution to this problem, the present research plans to elaborate a prototype of a computer application for the management of the hemovigilance system in the province of Pinar del Rio.
METHODS
A technological research-development study was carried out with the objective of elaborating a prototype of a computer application for the management of the hemovigilance system in the province of Pinar del Rio, applying the theoretical method, review of models and procedures for the definition of the informatics and empirical solution to observe in a planned way all the work developed in the Provincial Bank related to the hemovigilance system, using this method to evaluate the results and the impact of the proposed solution. Historical-logical to carry out a study of the state of the art that supports the research, inductive-deductive starting from the particularities of each stage of the information management process until defining in a general way all the requirements of the hemovigilance system, discussions and documentary analysis, to carry out a deep study about the evolution and development of the process.
Using the XP (Extreme Programming) development methodology: agile methodology based on continuous feedback between the client and the development team, which favors a good working environment, simplicity in the implemented solutions and courage to face changes. Unified Modeling Language (UML) to detail the artifacts in the system, elaborate use case diagrams, class diagrams, event flows, sequence diagrams and analyze their functionality. Enterprise Architect, a comprehensive UML design and analysis tool, which covers software development from the requirements step through the stages of analysis, design models, testing and maintenance, and softwareAxure RP, a tool for creating diagrams, wireframes, prototypes and specifications for websites, which generates html files and runs them in any browser.
The entire population of donors coming to the provincial blood bank was used as well as the components obtained from such donation, applying as acceptance and exclusion criteria provided by the workers of the center which are established as regulation in the work in blood banks.The application of scientific methods will allow the collection and processing of data from the normative and methodological documents in the department of the Provincial Bank, as well as other materials related to the object of information, will provide important elements for the theoretical foundations of the model and will allow deepening the historical background and the results of previously conducted research.
RESULTS
The mission of the Provincial Blood Bank is to obtain and guarantee the certified blood components that are delivered to the medical-pharmaceutical industry, and to the medical assistance for the assistance support to the patients admitted in our health institutions, with the objective of solving their problems and increasing the degree of satisfaction. As well as to supervise and guarantee the correct functioning of the Blood Program in the province of Pinar del Rio, whose maximum expression is the Hemovigilance System.
Product requirements.
The system must maintain the integrity of the information at all times.
The system must be available for use at any time as long as there is no maintenance work on the server.
The system code shall be properly commented to facilitate its maintenance.
The system shall not allow users to perform functions for which they are not authorized.
The developed prototype has the following requirements:
Functional Requirements.
During the interviews conducted, the following functional requirements to be implemented were defined:
Manage user
User authentication
Generate registration information
Manage donation information
Manage information in the laboratory process
Manage information in the production process
Manage information in the quality assurance process
Manage information in the distribution process
Generate reports regarding rejections of bags and blood components.
Generate transfusion request information
Generate transfusion process information
Generate reports of ineligibility for donation.
Generate adverse reaction to donation report
Generate adverse transfusion reaction reports
Generate statistical reports of components obtained
Generate statistical report of components sent to industry
Generate statistical report of dispatches made by services
Non-functional Requirements
Some of the non-functional requirements defined for the application development process are listed below.
Security
All steps or modifications must be saved with the name of the user performing the operation.
Interface
The donor's medical history model must meet the specifications defined by MINSAP.
The system must run smoothly and without errors when displaying the interfaces to users.
The system must present user interfaces that are easy to operate for each of the functions it performs.
The main interfaces of the prototype are presented below. These were designed in such a way that it would be possible to visualize them correctly regardless of the device used to access them.
Prototype Description
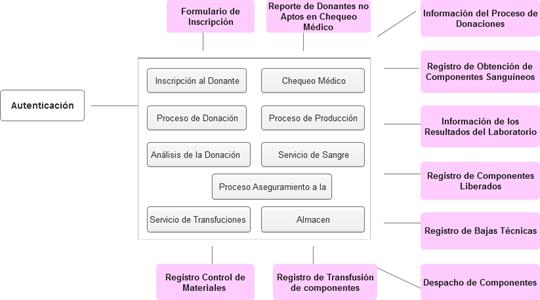
Prototype navigation diagram.
It illustrates how health professionals can navigate through the prototype and subsequently through the computer application (Fig. 1) Where the personnel must authenticate themselves with a user name and password previously registered in the system and then move through the different interfaces according to the user's assigned permissions for registration, donation, laboratory, production and quality assurance, the latter being responsible for the acceptance or rejection of the components obtained, The prototype is also designed to collect information from processes such as sending components to the medical-pharmaceutical industry and to the different transfusion services, as well as the transfusion process and possible adverse reaction to it, and the control and storage of indispensable material resources, although its interface is not shown.
The prototype created with the AxureRp tool is an html application that simulates the web application desired by the client. In this way, in the software design stage, the analysis with the final client of the functional requirements requested by him is facilitated, by responding to his needs before the programming of the final system is carried out.
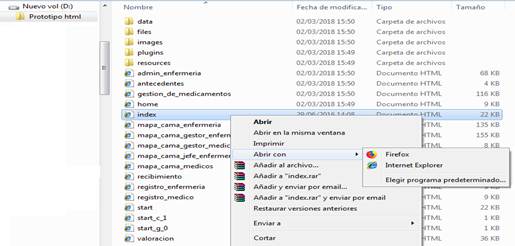
The html application will be conformed by a set of files, and it can be opened through the Explorer with the use of any browser (Fig. 2).
To access the prototype, the following steps must be followed:
1. Access in the Explorer to the folder where the html files are located (Fig. 2).
2. From the index, start or home files you can open the prototype. To do so, right click on the file and select Open with, (Fig. 3) and select the desired browser.
3. The prototype opens in the main view (index), for user authentication (Fig.4).
When the data (User and Password) are entered, the system will allow two options:
1. If the data entered is correct, it will allow access to the application modules that correspond to the role played by the system user, whether doctor, nurse or technologists, so that they can perform their functions.
2. If the data entered is incorrect, the system will issue the message: "Incorrect user and/or password", which invalidates the system login.
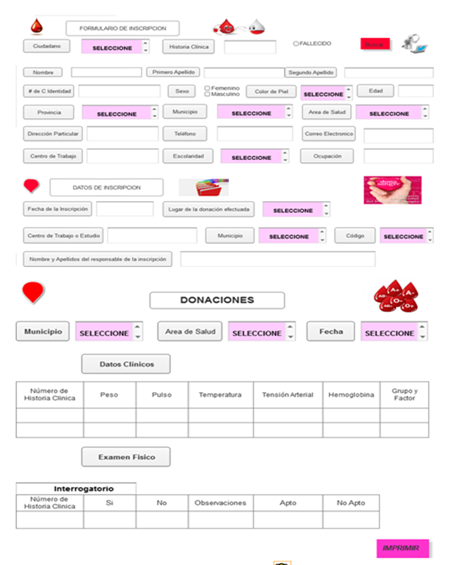
Users work with the application
Once the user is authenticated, he/she accesses the window of each of the modules defined by departments that will allow him/her to enter the information generated in each of the processes from registration, medical check-up with the general parameters measured in the same, identification of laboratory results, components obtained from each of the donations processed, identification of the release of such components either their rejection for not complying with the quality parameters or acceptance. (Fig.5,6)
DISCUSSION
With the increase in the development of informatics and communications, today many first world countries have the necessary infrastructure to automate all the processes that interact in the health services, thus achieving the challenge of digitalizing all the information in the different services provided to the population.
Cuba, being a country that pays a fundamental interest to the health of the population, makes multiple efforts to allocate the existing resources to increase the satisfaction of the services it provides and to improve the work environment for the professionals of the health sector, thus promoting the creation and updating of our computer applications that allow us to keep up with the times and the world's development.
When performing the processes included in the hemovigilance system, we must be very strict in collecting all the information derived from each procedure.
A group of software applications worldwide, intended for hemovigilance management, have been analyzed.
EdgeBlood is a software developed by the American company HaemoneticsCorporation Software Solutions as a comprehensive system to be used in blood banks regardless of their size. It was created to comprehensively manage the entire transfusion cycle from collection to follow-up of the recipients after transfusion.8
Based on the functionalities shown above, in principle it is the ideal solution to the problem posed. However, the costs associated with its operating license and technical support, together with the fact that its owner is a company registered in the United States and therefore governed by the laws of that country, make it impossible to use it within the framework of the Blood Program.8
SafeTraceTx is a system created by the aforementioned company Haemonetics Corporation Software Solutions to be used as an advanced transfusion management system. Designed to be used by transfusion units, it allows, among other functions:Electronic and remote cross-referencing of compatibility between patients and the component to be transfused. On-demand printing of ISBT 128 labels. Recording of quality controls performed on blood components. Generate detailed reports of transfusion histories, component usage. This software despite being feasible for transfusions has almost nothing related to donations which are an essential element within the hemovigilance system, being property of the mentioned company and the associated cost of licensing and technical support its main reasons for discarding it as an alternative to be used.8
Web System for Information Management at the Provincial Blood Bank, Pinar del Río: Assurance Module
It performs a complete analysis of the processes governed by the quality assurance department and its relationship with the laboratory and production departments, but does not reflect the other processes carried out at the Provincial Bank.9) Galen Blood Bank: The Galen Blood Bank is the only one of its kind in Pinar del Río.
Galen Blood Bank is a software developed by the Cuban company Softel focused on the management of the information generated in the blood banks during the blood donation process. It is currently used by the Provincial Blood Bank. Among its main functionalities are Donor registration. Blood certification. Medical check-up of the donor. Registration of the blood production process. Dispatch of blood components. Release of blood components. However, it presents a series of deficiencies in important aspects for blood program specialists: It does not have a module that allows statistical analysis. Incomplete reports or with information that is not completely correct. It does not have a module capable of managing the information related to the process of whole blood and blood components collection in fixed or mobile collection centers. It does not have a module capable of managing information regarding the hemovigilance process (adverse reactions to the donation process). In general, Galen Blood Bank is a software that fulfills many of the tasks necessary for the operation of the Blood Bank, but nevertheless the deficiencies it presents hinder the work of the personnel who must use it, causing their work to be delayed, and therefore it is sometimes not used.9,10
The existing solutions analyzed previously do not meet the requirements to be used by the blood program in the province of Pinar del Rio since they do not fulfill the indispensable requirements included in a hemovigilance system. Therefore, it was decided to develop the research to propose the information architecture that will allow obtaining, in the future, a computer application for the management of this system in the province, obeying the planning and design stages with the use of the XP software development methodology.
CONCLUSIONS
A prototype of a computer application for the management of the hemovigilance system in the province of Pinar del Rio was elaborated, which will provide programmers with the requirements for its later implementation as a tool that enables health professionals to obtain the necessary information about the Hemovigilance System in the province of Pinar del Rio and to speed up the daily work in the processes that make up this system, making the processing and storage of the information generated in it more reliable and pleasant. Although the prototype is based on the hemovigilance system in Pinar del Rio province, it can be used by other health care units since each of its interfaces is based on what is defined by the National Blood Program. The implementation of this tool would save material resources, would increase workers' satisfaction since it would reduce the time required to process and obtain vital information and would increase the control of the entire hemovigilance system from the highest level. The interviewees reported satisfaction with the prototype computer application.
BIBLIOGRAPHIC REFERENCES
1. OPS/OMS. Guía para establecer un sistema nacional de HV [Internet]. Washinton; 31 marzo 2017. [citado 15/02/2018]. Disponible en: Disponible en: http://www.paho.org/hq/index.php?option=com_content&view=article&id=13044%3Agui-hemovigilancia-2017&catid=4669%3Aannouncements-hss&Itemid=39594&lang=es 1. . [ Links ]
2. Silva-Ballester HM, Bencomo-Hernández A, Benet CM, López-Fernández R, Valls W, Ballester Santovenia JM. Una experiencia pionera en la Hemovigilanciacubana. SETS [Internet]. 2014 [citado 26/06/2017]; 26(3): 21-5. Disponible en: Disponible en: http://www.sets.es/index.php/cursos/biblioteca-virtual/boletin-sets/401-boletin-sets-90-2014/file 2. [ Links ]
3. Martínez-Abreu J, de-Léon-Rosales L, García-Herrera A, Betancourt-Pérez-Carrion N. Desarrollo de la informatización en la Universidad de Ciencias Médicas de Matanzas. Revista Médica Electrónica [Internet]. 2018 [citado 06/02/2023]; 40(6): 1724-1728. Disponible en: Disponible en: https://revmedicaelectronica.sld.cu/index.php/rme/article/view/3046 3. [ Links ]
4. Bravo-Lindoro AG. Hemovigilancia y transfusión en México. RevHematolMex [Internet]. 2018[citado 06/02/2023]; 19(3): 105-108. Disponible en: Disponible en: https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=83490 4. [ Links ]
5. Lucidchart. Qué es el lenguaje unificado de modelado (UML) [Internet].Lucidchart; 2017[actualizado 26 marzo 2019; citado 23/04/2019]. Disponible en: Disponible en: https://www.lucidchart.com/pages/es/que-es-el-lenguaje-unificado-de-modelado-uml 5. [ Links ]
6. Ionos. UML, lenguaje de modelado gráfico. [Internet].Ionos; 2018[actualizado 26 marzo 2019; citado 23/04/2019]. Disponible en: Disponible en: https://www.ionos.es/digitalguide/paginas-web/desarrollo-web/uml-lenguaje-unificado-de-modelado-orientado-a-objetos/ 6. [ Links ]
7. García MC. Ética y calidad en los servicios de sangre. Acta Bioética [Internet]. 2011 [citado 08/02/2019]; 17(1). Disponible en:Disponible en:https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S1726-569X2011000100007 7. [ Links ]
8. Monduy OL. Expediente Maestro Banco de Sangre Provincial; 2019. [ Links ]
9. Guía para establecer sistema nacional de hemovigilancia. Washintong, DC; 2017. [ Links ]
10. Macia Fonseca E. Trabajo de Diploma [Internet]. CUBA: Universidad central Martha Abreu de las Villas; 2019 [citado 17/03/2021]. 115 p. Disponible en: Disponible en: https://dspace.uclv.edu.cu/bitstream/handle/123456789/7273/Macia%20Fonseca%2C%20Eilenis.pdf?sequence=1&isAllowed=y 10. [ Links ]
Received: June 04, 2022; Accepted: January 19, 2023











 texto en
texto en