Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Ciencias Médicas de Pinar del Río
versión On-line ISSN 1561-3194
Rev Ciencias Médicas vol.27 supl.1 Pinar del Río 2023 Epub 01-Jul-2023
Articles
Level of knowledge of dental students on the use and storage of sodium hypochlorite
1Regional Autonomous University of the Andes (UNIANDES). Ecuador.
ABSTRACT Introduction: sodium hypochlorite is considered the most widely used irrigant solution worldwide, its characteristics and low cost make it to be considered the "Gold standard" as a complement for endodontic instrumentation. Objective: io determine the knowledge of dental students in their seventh to tenth semesters regarding the handling and storage of sodium hypochlorite prior to the beginning of their pre-professional internships. Methods: an observational, descriptive, cross-sectional, descriptive study was carried out in the academic period May - September 2022, in students from eighth to tenth semester of the dentistry career of the Regional Autonomous University of the Andes "UNIANDES" on their knowledge about the storage of sodium hypochlorite, as well as the concentrations of preference and the cases in which each one of them would be used. An opinion poll was used to find out the most frequent storage conditions, with five closed and multiple choice questions, the variables used and the results obtained were described statistically by means of percentages. Results: 36,8 % of the students do not store sodium hypochlorite correctly; they use containers that do not protect the irrigant from ambient light. 97,4 % of the respondents have the knowledge about the environmental condition of storage and temperature. There is a discrepancy regarding the concentration that should be used for the instrumentation of pulps with a diagnosis of necrosis, since 50,4 % use higher concentrations (5,25 %) while 42,7 % use 2,5 % irrigant. Conclusions: the research should be complemented with experimental studies in which it can be demonstrated that storage conditions can impair the composition and stability of sodium hypochlorite.
Key words: SODIUM HYPOCHLORITE; STORAGE; CONCENTRATION; TEMPERATURE.
INTRODUCTION
Sodium hypochlorite (NaOCl) is an irrigant widely used in endodontic therapy as it is a solvent of organic and inorganic tissue aided by instrumentation, in addition to its bactericidal effect against a wide range of microorganisms found in the microbiota of root canals (bacteria, fungi, viral forms).1)
NaOCl is a chemical solution with high alkalinity (pH 11-12.5); it is a protein oxidizing agent and highly hemolytic when in contact with red blood cells, even in its lowest concentrations (1:1 000), it is irritating and can cause ulcerations in skin, mucous membranes and cornea, which makes it a potential generator of cystic necrosis due to its ability to dissolve organic tissue.2
As an irrigant agent, NaOCl continues to be the first choice; its concentration varies from 0,5 % to 6 %, being 5,25 % the most used concentration due to its great potential and rapidity in dissolving organic tissue, but at the same time, greater cytotoxicity. Some clinicians do not recommend this concentration because of its irritant effect on periapical tissues; however, using it at 0,5 % is not enough to act on some microorganisms such as Staphylococcus aureus or Enterococcus faecalis.3
Sodium hypochlorite has been defined by the American Association of Endodontics as a clear, pale, yellowish-green, extremely alkaline liquid with a strong chlorine odor, which has a dissolving action on necrotic tissue and organic debris, besides being a potent antimicrobial agent.4
During 1915 in World War I, Dakin introduced sodium hypochlorite solution in a concentration of 0,45 % to 0,50 % for disinfection of open and infected wounds.5) In 1917 Barret spread the use of Dakin's solution in dentistry, especially for irrigation of root canals and reported the efficiency of the solution as an antiseptic.6
Years later, Coolidge used sodium hypochlorite to improve the process of cleaning and disinfection of root canals.7) One of the pioneers in the use of 5,0 % sodium hypochlorite as a solvent of organic matter and potent germicide was Dr. Blass. Blass; his experiences were published in the Fifth Edition of the National Formulary; Walker in 1936 refers to the use of 5,0 % sodium hypochlorite in the preparation of root canals of teeth with necrotic pulps.8
Although sodium hypochlorite is widely used in endodontics, there is still no consensus on the ideal concentration. Frequent and copious irrigation with a 2,5 % sodium hypochlorite solution can maintain a sufficient reserve of chlorine to eliminate a significant number of bacterial cells, compensating for the irritating effect caused by the use of high concentrations.9
Therefore, we established as the objective of the present investigation to determine the knowledge of dental students from seventh to tenth semester regarding the handling and storage of sodium hypochlorite prior to the beginning of their preprofessional practices.
METHODS
An observational, descriptive, cross-sectional, cross-sectional study was carried out in the academic period May - September 2022, in students from eighth to tenth semester of the dental career of the Regional Autonomous University of the Andes "UNIANDES" on their knowledge about the storage of sodium hypochlorite, as well as the concentrations of preference and the cases in which each one of them would be used.
An opinion survey was used to find out the most frequent storage conditions, with five closed and multiple choice questions, the variables used and the results obtained were described statistically by means of percentages.
The research approach of this article is mixed: qualitative-quantitative, the qualitative aspect is included specifically because it determines the level of knowledge about the handling and storage of sodium hypochlorite of students from eighth to tenth semester of the dentistry career of the Uniandes University.
According to its purpose, it is an applied research because through the study, concepts and recommendations for the storage of sodium hypochlorite are established and strengthened.
The descriptive scope of the research was carried out by means of a survey, using Google Forms, software in charge of the administration of surveys and analysis of results. The questionnaire was designed by gathering all the variables that affect the pH of sodium hypochlorite, to be extrapolated to seven closed polytomous questions that describe the storage conditions of sodium hypochlorite.
The sample was taken at convenience, based mainly on criteria of knowledge in the endodontics course and use of sodium hypochlorite in preprofessional practices. All eighth, ninth and tenth semester students of the Dentistry course of the Regional Autonomous University of the Andes were included. The link to the survey was sent digitally through their course representatives.
This article analyzes the criteria of the eighth to tenth semester students of the dentistry career of the Regional Autonomous University of the Andes "UNIANDES", in the academic period May - September 2022, on their knowledge about the Gold-standard storage of endodontic irrigation, as well as the concentrations of preference and the cases in which each one of them would be used.
RESULTS
From the data collection of the Google Forms software, 117 responses were obtained from eighth to tenth semester dental students.
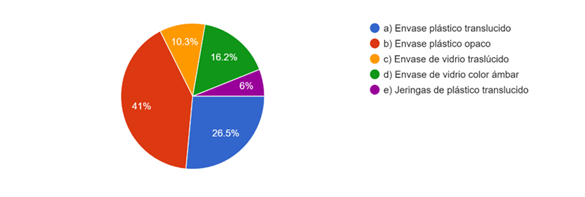
Forty-one percent of the respondents store sodium hypochlorite in an opaque plastic container, while 26,5 % of the students use translucent plastic containers, impairing the stability of the available chlorine molecule; followed by 16,2 % in amber glass containers and 10,3 % in translucent glass containers (Fig. 1).
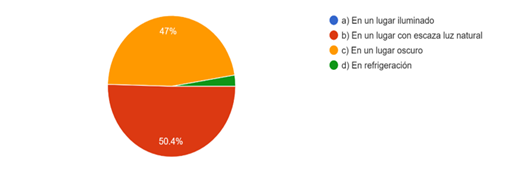
50,4 % of the responses received consider that sodium hypochlorite needs to be handled in an environment with little natural light while 47 % affirm that a dark place is ideal for storage, only 2,6 % keep sodium hypochlorite in refrigeration, where light and heat are avoided at the same time, reducing the degree of evaporation, as long as the freezing point is not reached (Fig. 2).
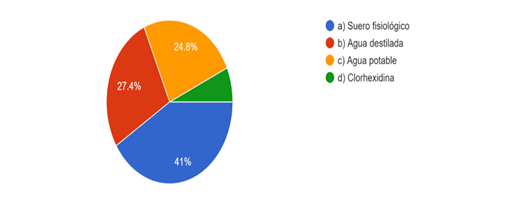
Forty-one percent of the respondents dilute the solution with the use of saline solution; 27,4 % with distilled water and 24,8 % with potable water. All options are scientifically accepted. A minority of 6,8 % dilute with chlorhexidine, which is not considered a viable option due to the formation of precipitate. (Fig. 3)
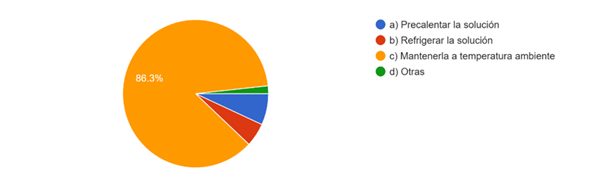
86.3% consider that room temperature sodium hypochlorite is ideal for use in the irrigation protocol as specified in question 4, 6,8 % preheat the sodium hypochlorite, 5,1 % keep under refrigeration before irrigating the ducts. (Fig. 4).
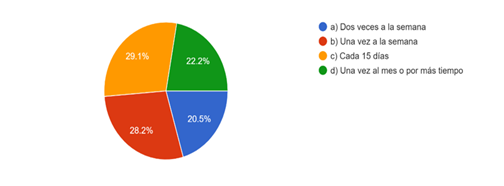
There are different criteria of the students regarding the change and renewal of solution, where after 15 days 29,1 % of respondents do it; while 28,2 % do it weekly, 20,5 % twice a week. The 22,2 % change the sodium hypochlorite solution once a month or leave it for longer, which if not kept in a suitable environment (fresh and with little light) would reduce the available chlorine below acceptable levels. (Fig. 5)
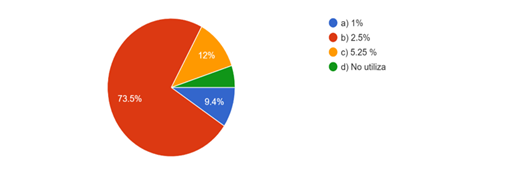
One of the fundamental pillars of irrigation and instrumentation is the previous diagnosis, where 73,5 % use sodium hypochlorite at 2,5 % to perform biopulpectomies, 12 % at a concentration of 5,25 %; 9,4 % consider that 1% is an adequate condition for the aforementioned therapeutics (Fig. 6).
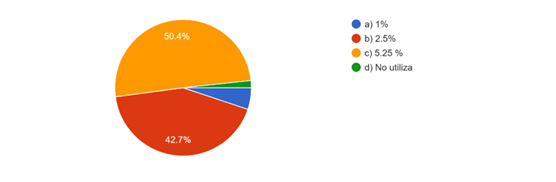
In necropulpectomies 50,4 % use sodium hypochlorite at 5,25 % followed by 42,7 % at 2,5 % while 5,1 % use sodium hypochlorite at 1%. (Fig. 7)
DISCUSSION
The 26,5 % of the students use translucent plastic containers as well as 10,3 % in glass containers with the same characteristics for the storage of sodium hypochlorite, where the available chlorine molecule is affected because it is photosensitive;8) one of the classic studies in the endodontic literature mentions that factors such as the storage container, temperature, and time impair the stability of the solution, in the mentioned study a 12-month follow-up of the different concentrations was carried out, showing a decrease in the properties after the established time, it should be emphasized that in the study the constant variable was the protection to photosensitivity, using plastic and glass containers that do not allow the passage of light, therefore emphasis should be placed on the correct handling of the storage conditions of the students.9
97,4 % of the respondents affirm that ambient light can be harmful for sodium hypochlorite since they consider that dark places or places with little light are ideal to keep it, this result has discrepancy with what was obtained in the characteristics of the container, since it is demonstrated that there is concern on the part of the respondents at the moment of storing the irrigant but not in what type of container should be used.
2.6% keeps sodium hypochlorite refrigerated, where light and heat are avoided, and the available chlorine molecule is kept stable, in the study by Nandakumar et al,10) it was shown that the use of cryotherapy with the irrigant refrigerated at four degrees controls post-endodontic pain in daily clinical practice, which benefits the treatment.
The temperature at which the irrigant is stored also plays an important role in the stability of the available chlorine molecule, concentration and pH. In the study it was shown that after 200 days of storage, the 5 % sodium hypochlorite solution showed greater decomposition in terms of its composition when stored at a room temperature of 24 degrees compared to the solution stored at four degrees.9
In the present study it was reported that 73,5 % of the students use sodium hypochlorite at (2,5 %), 12 % at (5,25 %); 9. In the study of Verma et al,11) it is established that there is no statistically significant difference between using any of the concentrations for the recovery of periapical tissue post-treatment, the effectiveness of dissolution of tissue and organic matter depends on several factors, but mainly on the concentration, temperature, flow, ultrasonic activation.12)
Sodium hypochlorite with low concentrations can be more effective if the variables mentioned above are added. Stojicic et al,13) in their study, compared the effectiveness of different concentrations of sodium hypochlorite (1 %,2 %,4 %,5,8 %) to dissolve pulp tissue, in their methodology they included the determination of mass lost after immersing the samples in the different concentrations, it was demonstrated that with the addition of ultrasonic activation the effectiveness of dissolution of the lower concentrations is enhanced, becoming comparable with the higher ones.
Root resorption is common in cases of pulp necrosis together with resorption of the apical bone, in these cases the apical constriction is lost, so it is very likely that the apical foramina are permeable, for this reason, there should be an exhaustive irrigation protocol, but controlled to avoid a possible accident with sodium hypochlorite.14,15) There is much discrepancy in the literature about the ideal concentration for endodontic irrigation, the respondents use higher concentrations (5,25 %) in necropulpectomies in 50,4 % while 42,7 % at 2,5 %, the choice of concentration is multifactorial and depends entirely on the clinician.
There are studies in which it is determined that the flow of the irrigant through the needle is also very important, the flow pattern of the open needles is greater than those with a closed design, which results in a greater replacement of the irrigant solution, but it is possible that there is greater apical pressure and therefore an extrusion of irrigant towards the periradicular tissues.16
One of the variables reported in the studies is the Ph of the irrigant, which can be enhanced to obtain a better dissolution of organic tissue,17,18) the present investigation raises the question of whether the storage conditions can alter this variable.
CONCLUSIONS
The concentrations of sodium hypochlorite used in practice for root canal irrigation are not the most effective according to the results of this research. The concentrations of hypochlorite in commonly used commercial products are not the concentrations commended in the literature 5,25 w/v and 2,5 % w/v; this may cause tissue damage when hypochlorite solutions are irrigated inadequately and without isolation.
BIBLIOGRAPHIC REFERENCES
1. Zhu WC, Gyamfi J, Niu LN, Schoeffel GJ, Liu SY, Santarcangelo F, et al. Anatomy of sodium hypochlorite accidents involving facial ecchymosis - a review. Journal of Dentistry [Internet]. 2013 [Citado 1/05/2022]; 41(11):935-48. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/23994710/ 1. [ Links ]
2. Bartolo A, Koyess E, Camilleri J, Micallef C. Model assessing thermal changes during high temperature root canal irrigation. Healthcare Technology Letters [Internet]. 2016 [Citado 1/05/2022]; 3(3): 247-51. Disponible en: Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5047292/ [ Links ]
3. Baser Can ED, Karapinar Kazandag M, Kaptan RF. Inadvertent apical extrusion of sodium hypochlorite with evaluation by dental volumetric tomography. Case Reports in Dentistry [Internet]. 2015 [Citado 1/05/2022]; 2015: 247547. Disponible en: Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4390169/ 3. [ Links ]
4. Glossary: American Association of Endodontics. Contemporary terminology for endodontics. 6th ed. Chicago; 1998. [ Links ]
5. Dakin HD. On the use of certain antiseptic substances in the treatment of infected wounds. Br Med J [Internet]. 1915 [Citado 1/05/2022]; 2(2852): 318-320. Disponible en: Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2303023/ 5. [ Links ]
6. Barret MT. The Dakin-carrel antiseptic solution. Dent Cosmos [Internet]. 1917 May [ Citado 1/05/2022]; 71(5): 237-240. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/33703281/ 6. [ Links ]
7. Coolidge ED. Studies of germicides for the treatment of root canals. J Ame Dent Assoc [Internet]. 1929 [Citado 1/05/2022]; 16(4): 698-712. Disponible en: Disponible en: https://www.sciencedirect.com/science/article/abs/pii/S1048636429640165 7. [ Links ]
8. Walker A. A definite and dependable therapy for pulpless teeth. J Ame Dent Assoc [Internet]. 1936 [Citado 1/05/2022]; 23(8): 1418-1425. Disponible en: Disponible en: https://www.sciencedirect.com/sdfe/pdf/download/eid/1-s2.0-S1048636436380038/first-page-pdf 8. [ Links ]
9. Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J [Internet]. 1985 [Citado 1/05/2022]; 18(1): 35-40. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/3922900/ 9. [ Links ]
10. Nandakumar M, Nasim I. Effect of intracanal cryotreated sodium hypochlorite on postoperative pain after root canal treatment - A randomized controlled clinical trial. J Conserv Dent [Internet]. 2020 [citado 11/11/2022]; 23(2): 131-6. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/33384483/ 10. [ Links ]
11. Verma N, Sangwan P, Tewari S, Duhan J. Effect of Different Concentrations of Sodium Hypochlorite on Outcome of Primary Root Canal Treatment: A Randomized Controlled Trial. J Endod [Internet]. abril de 2019 [citado 12/11/2022]; 45(4): 357-63. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/30827769/ 11. . [ Links ]
12. Del Carpio-Perochena A, Monteiro Bramante C, Hungaro Duarte M, Bombarda de Andrade F, Zardin Graeff M, Marciano da Silva M, et al. Effect of Temperature, Concentration and Contact Time of Sodium Hypochlorite on the Treatment and Revitalization of Oral Biofilms. J Dent Res Dent Clin Dent Prospects [Internet]. 2015 [citado 17/11/2022]; 9(4): 209-15. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/26889356/ 12. [ Links ]
13. Stojicic S, Zivkovic S, Qian W, Zhang H, Haapasalo M. Tissue dissolution by sodium hypochlorite: effect of concentration, temperature, agitation, and surfactant. J Endod [Internet]. septiembre de 2010 [citado 21/11/2022]; 36(9): 1558-62. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/20728727/ 13. . [ Links ]
14. Gambarini G, De Luca M, Gerosa R. Chemical stability of heated sodium hypochlorite endodontic irrigants. J Endod [Internet]. junio de 1998 [citado 11/11/2022]; 24(6): 432-4. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/9693589/ 14. [ Links ]
15. Wright PP, Kahler B, Walsh LJ. The Effect of Heating to Intracanal Temperature on the Stability of Sodium Hypochlorite Admixed with Etidronate or EDTA for Continuous Chelation. J Endod [Internet]. enero de 2019 [citado 21/11/2022]; 45(1): 57-61. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/30446402/ 15. . [ Links ]
16. Berman LH, Hargreaves KM. Cohen’s Pathways of the Pulp - 12th Edition [Internet]. 12.a16. ed. ELSEVIER; 2020 [citado 10/11/2022]. Disponible en: Disponible en: https://www.elsevier.com/books/cohens-pathways-of-the-pulp/berman/978-0-323-67303-7 16. [ Links ]
17. Claudino Ribeiro JR, da Silveira Bueno CE, Bruno KF, Dos Reis S, de Martin AS, Fontana CE, et al. Impact of Sodium Hypochlorite on Organic Tissue Dissolution in the Periapical Region of Immature Permanent Teeth: An Ex Vivo Study. J Endod [Internet]. abril de 2022 [citado 10/11/2022]; 48(4): 555-60. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/35032539/ 17. . [ Links ]
18. Córdova Lascano AC, Ortiz Suárez HS. Usos del dióxido de cloro como prevención y tratamiento de la COVID-19 desde la percepción del usuario. Salud Cienc. Tecnol [Internet]. 2022 [citado 10/11/2022]; 2(S1): 176. Disponible en: Disponible en: https://doi.org/10.56294/saludcyt2022176 18. [ Links ]
Received: April 19, 2023; Accepted: April 24, 2023











 texto en
texto en