Introduction
The pharmaceutical industry is a priority sector of society due to the goods it generates, which are essential because they make possible vital improvements in health and, therefore, in the well-being and quality of life of citizens. Thus, it has a highly responsible social mission, which must be carried out with a high degree of exigency in the quality of its productions (Durrant, 2001).
The oral route is the most widely used for drug administration since it has undoubted advantages due to its simplicity, safety, and convenience. Within the formulations for oral administration, we have: solid, semisolid, and liquid being the most common drugs in solid pharmaceutical forms such as tablets and capsules due to the greater productivity of their processes, high stability, storage, and transport facilities, among others. However, they are not helpful for administration in pediatric, geriatric, or dysphagic patients due to their inability of these to swallow them (Thabet, 2018).
Despite regulatory advances in pediatrics, most active ingredients are marketed only as solid dosage forms or in higher doses than necessary for the pediatric population; some of these drugs are Sildenafil, spironolactone, Furosemide, Methadone, Phenobarbital (Provenza, 2014). For these patients, there remains the option of elaborating easy-to-swallow formulations, which is why there are suspensions either as aqueous dispersed systems or as mixtures of powders to reconstitute extemporaneously. This variant allows more excellent stability (Batchelor, 2015).
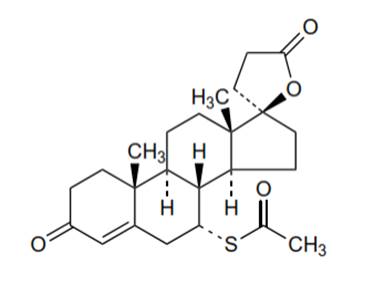
Spironolactone, a synthetic steroid, competitive aldosterone antagonist (Figure 1) that acts in the distal renal tubules to increase sodium and water elimination and reduce potassium elimination, is manufactured and marketed. The use of this molecule in children was first reported in 1964 when the drug was administered in three infants producing moderate diuresis. Since then, spironolactone has been widely used to manage congestive heart failure associated with congenital heart disease, bronchopulmonary dysplasia or chronic disease, and pediatric ascites (Walker, 1964).
Spironolactone is marketed in tablet form in doses of 25 and 100 mg and indicated as a diuretic in numerous pathologies, especially in adults; however, to date, there is no marketed pharmaceutical form of this drug for pediatric therapy (National Center for Biotechnology Information, 2022).
 Source: National Center for Biotechnology Information, (2022)
Source: National Center for Biotechnology Information, (2022)
Fig. 1 - Chemical structure of spironolactone
In this context, a preliminary study of a possible formulation of a powder for suspension (5mg/mL) for pediatric use was carried out, to which the following tests were performed: i) sedimentation time, ii) pH measurement, iii) determination of particle size, polydispersion, and Z potential, iv) rheology and viscosity, v) accelerated stability by TurbiscanTMLab, vi) quantification of the active principle, vii) dissolution test and viii) microbiological tests.
Materials and methods
Spironolactone reference standard, Acofarma (Barcelona, Spain), and spironolactone raw material (Tianjin Harmony Pharmaceutical Co. China) were used. The excipients used were: xanthan gum, carboxymethylcellulose (CMC), sodium saccharin, potassium sorbate, sorbic acid, colloidal silicon dioxide, saccharose powder, vanilla flavor, all obtained from Distribuidora Caribe (Tegucigalpa, Honduras). Methanol, acetonitrile HPLC grade J.T. Baker Thermo Fisher Scientific (Waltham, Massachusetts, USA), and ultrapure water using the Milli-Q® System.
Method of preparation of spironolactone suspensions
Three spironolactone powder formulations were prepared to obtain a test batch of 10 vials. The formulations were named: F1, F2, and F3. The powders were sieved through a sieve with a mesh opening of 75µm (US Standard Sive, USA), after which these powders were weighed in an analytical balance (Ohaus explorer Pro, Mexico City, Mexico) and then mixed manually in a cylindrical stainless-steel container, applying an oscillating movement (in eight) for five minutes, using geometric mixing. The content by weight of each bottle was defined, which were filled based on the weight of powder necessary to contain the desired dose and then hermetically sealed in PVC bottles 10.50 cm high and 15 cm wide with a maximum capacity of 100 mL to store up to a maximum of 60 mL of formulation (Diamond Plastics, Tegucigalpa, Honduras). F1 contained CMC as suspending agent, and F2 and F3 had xanthan gum as a suspending agent in different proportions.
Physicochemical analysis of spironolactone suspensions.
Sedimentation time
Thirty mL of each formulation (F1, F2, and F3) were placed in a test tube. The time taken for each of the suspensions to settle was observed. These were monitored every hour for 24 hours.
pH measurement
The pH measurement was carried out by taking 20 mL of each formulation and using a pH meter (Crison Instruments S.A., Barcelona, Spain) by directly immersing the electrode in the samples. The pH values were measured at 15 and 60 minutes after reconstitution.
Rheological study
The rheological study of each formulation was carried out with a Thermo Scientific Haake Rheostress 1 rheoviscosimeter (Thermo Fischer Scientific, Karlsruhe, Germany) equipped with a plate-plate system. The rheometer used in rotational mode allowed for obtaining the viscosity and flow curves (n=2). For this purpose, each sample of spironolactone powder reconstituted with water was subjected to a shear program including a. A rising velocity section from 0 to 100 s-1 for 3 min, b. A section at a constant velocity of 100 s-1 for 1 min and c. A downward leg from 100 s-1 to 0 s-1 for 3 minutes at a temperature of 25°C.
Particle size determination
To determine the particle size at 25°C of each of the spironolactone suspension formulations, a 1/10 dilution was performed, and the measurement was made with a Malvern Master Sizer Malvern (Malvern Instruments, Worcestershire, UK).
Polydispersion and Z-potential
The Polydispersion (PDI) and Z-potential values of the three spironolactone suspension formulations were determined at 25ºC, making a 1/10 dilution, and the dynamic light scattering (DLS) method was used using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) (n=3).
Accelerated stability prediction using TurbiscanTM Lab
After choosing the best formulation according to its rheological behavior, viscosity, particle size, PDI, and Z-potential, the accelerated stability study was performed using by DLS technique, using a TurbiscanTM Lab (Formulation Co., L'union, France). The method determines the physical stability, i.e., it gives us a quick prediction as to whether the drug will undergo precipitation, flocculation/coalescence, and suspension of particles on the surface or rinsing. The lines generated after the assay and superimposed indicate that the formulation will be physically stable for at least six months. Variations of 10% are acceptable. The ¨Backscattering¨ indicator is used for opaque formulations, and the ¨transmission¨ indicator is used for precise formulations. For this formulation, we use the Backscattering measurement generated by the apparatus (Provenza, 2014).
Quantification of spironolactone by High-Performance Liquid Chromatography (HPLC).
The analytical methodology by which spironolactone was quantified on different working days (days 0, 15, and 30) and stored at various temperatures (4, 25, 40°C) using the USP 35 monograph of spironolactone is described below. A High-Resolution Liquid Chromatograph (Shimadzu, Prominence) was used.
Solvent: Acetonitrile: water (1:1).
Standard Preparation. 25 mg of spironolactone Standard of reference Acofarma (Barcelona, Spain) was accurately weighed in a 50 mL flask, 25 mL of solvent was added, sonicated until complete dissolution, and made up to volume to obtain a concentration of 0.5 mg/mL. A portion of this solution was passed through a filter with a pore size of 0.45 µm, and the first 5 mL of the filtrate was discarded.
Titration preparation. The 5 mg equivalent of spironolactone from the suspension powder was weighed and transferred to a 10 mL volumetric flask; approximately 5 mL of solvent was added, sonicated for 10 minutes, made up to volume, and mixed. A portion of this solution was passed through a filter with a pore size of 0.45 µm, and the first 5 mL of the filtrate was discarded.
Mobile Phase: Methanol: Water (60:40)
Chromatographic system.
The High-Performance Liquid Chromatograph was equipped with a detector at 230 nm and a 4.6 mm x 150 mm column packed with L1 material (Column Code: C18-02-14); the flow rate was set at approximately 1 mL per minute with a temperature of 35°C. Equal volumes (about 10 µL) of the standard and titration preparation were injected separately into the chromatograph, the chromatograms were recorded, and the responses corresponding to the significant peaks were measured. Calculations were considered the calibration curve performed on the same day of the assay by linear regression using Prism Software version 3.00 (Graphpad Software Inc. San Diego, CA, USA).
Spironolactone dissolution kinetics.
A dissolution test was performed according to the parameters established in USP 35, taking into account the following criteria:
Dissolution medium: 0.1 N hydrochloric acid/ 0.1% sodium lauryl sulfate.
rpm: 75
Time: 60 min
Apparatus: 2
Volume: 900 mL.
The methodology used for quantifying spironolactone was performed by UV-VIS spectrophotometry, a methodology previously validated in the study of Provenza et al. (Provenza, 2014). Two dissolution tests were performed, one test with Aldactone® commercial tablets to compare the dissolution rate with the spironolactone suspension chosen as the best formulation. Aliquots of 5 mL were taken at different times: 2, 5, 8, 12, 15, 20, 25, 30, 40, 50 and 60 minutes for the suspension and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and 60 minutes for the tablets. The calculations took into account the calibration curve performed on the same day of the assay, using linear regression using Prism Software version 3.00 (Graphpad Software Inc. San Diego, CA, USA).
Microbiological control of the spironolactone suspension
For the microbial count, a 1:10 dilution of the F2 was performed, using 90 mL of peptonized water pH 7, to which 10 mL of the spironolactone 5 mg/mL suspension was added to obtain a concentration of 10-1. Then, 100 µL of the dilution was seeded in triplicate on plates with different culture media.
The culture media were 3: PDA (potato dextrose agar, for fungi and yeasts), SMA standard agar (Standard Method Agar, for mesophilic aerobic microorganisms), VRBA (neutral red-red-violet-crystal bile agar, for Gram-negative microorganisms). A neutralizing or inactivating agent, tween 80, was added to the plates with the culture media to inactivate the preservative and inhibit its biocidal action. This prevented the preservatives from distorting the interpretation of the results. Subsequently, the SMA and VRBA plates were incubated for 24 and 48 hours at 37°C; an observation was made at the end of the day and then incubated again for another 24 hours. PDA plates were stored in a closed container at room temperature for one week.
Microbiological analysis for isolation of mesophilic aerobic microorganisms, total coliforms, molds, and yeasts was evaluated at 0, 15, and 30 days and temperatures of 6°C, monitored environment (30°C) and 40°C, with a variation of ± 2°C.
Acceptance limit: In non-sterile oral liquid suspensions, aerobic mesophilic organisms allowed up to 102 CFU/mL, filamentous fungi and yeasts up to 101 CFU/mL, and the absence of Escherichia coli (USP,2012).
Evaluation of the disinfection process of the container.
The primary container (bottle) was analyzed to determine whether the 0.1% benzalkonium chloride with which it was disinfected fulfilled its function. For the microbiological analysis, 0.1% benzalkonium chloride was prepared. Then three containers were labeled for the test, adding a 1/10 dilution of Bacillus subtilis spores. They were capped and shaken, and the residues of the Bacillus subtilis spores were removed.
The indicator bacillus residues were removed. Afterward, each bottle was washed with distilled water and neutral soap and disinfected with benzalkonium chloride (0.1%). Once the bottles were cleaned, they were swabbed, seeded on 5% blood gelose plates, and incubated at 37°C for 24 hours (USP, 2012).
Statistical analysis. The values of means, standard deviations, and linear regressions (the latter precisely to calculate the drug content in the suspensions and the dissolution test) were obtained using the GraPhad version 5.0 program. The values of viscosity, thixotropy, rheological behavior, particle size, polydispersion, and Z potential were generated directly by the software attached to the apparatus.
Results
Preparation of suspensions
Three formulations of spironolactone suspension were prepared for subsequent reconstitution with water. Two different suspending agents were used; CMC for F1 and xanthan gum for F2 and F3, the latter in two different concentrations, 100 mg, and 150 mg, respectively (see Table 1). To obtain a test batch of 10 vials per formulation, 81,835 mg (81.83 g) of the mixture (F1 and F2) and 81,335 mg (81.33 g) of the mixture (F3) were weighed.
For each vial, 8,183.50 mg (8.18 g) of the mixture of F1 and F2 and 8,133.50 mg (8.13 g) of batch F3 were weighed. The powders were then reconstituted with ultrapure water to obtain 50 mL of spironolactone suspension at a final concentration of 5 mg/mL. The vials were filled manually by weighing the powder on an analytical balance.
The formulations after reconstitution with water were white in color, milky in appearance (see Figure 2), and somewhat viscous.
Table 1 - The final composition of the three spironolactone formulations expressed in mg and percentage (%).
| Number | Components | F1 (mg) | F1 (%) | F2(mg) | F2 (%) | F3(mg) | F3 (%) |
|---|---|---|---|---|---|---|---|
| 1 | spironolactone | 250 | 3.05 | 250 | 3.05 | 250 | 3.07 |
| 2 | CMC | 150 | 1.83 | 0 | 0.00 | 0 | 0.00 |
| 3 | Xanthan Gum | 0 | 0.00 | 150 | 1.83 | 100 | 1.23 |
| 4 | Sorbic acid | 100 | 1.22 | 100 | 1.22 | 100 | 1.23 |
| 5 | Potassium sorbate | 56 | 0.68 | 56 | 0.68 | 56 | 0.69 |
| 6 | Powdered cherry flavor | 60 | 0.73 | 60 | 0.73 | 60 | 0.74 |
| 7 | Colloidal silicon dioxide | 500 | 6.11 | 500 | 6.11 | 500 | 6.15 |
| 3 | Sodium saccharin | 67.50 | 0.82 | 67.50 | 0.82 | 67.50 | 0.83 |
| 8 | Sucrose | 7000 | 85.54 | 7000 | 85.54 | 7000 | 86.06 |
| Total | 8183.50 | 100.00 | 8183.50 | 100.00 | 8133.50 | 100.00 | |
Sedimentation time
The sedimentation time of F1 was 30 minutes, and F2 and F3 did not sediment for more than 24 hours. Figure 2 shows the images of the three formulations after the study time.

Fig.2- A - Formulation 1 (with CMC suspending agent). B. Formulation 2 (with suspending agent xanthan gum 150 mg). C. Formulation 3 (with suspending agent xanthan gum 100 mg).
pH analysis
The pH values at 25ºC of F1, F2, and F3 at 15 and 60 minutes after reconstitution of the suspensions can be observed in Table 2.
Table 2 - pH values of the three formulations of spironolactone suspension.
| Formula | pH (15 min) | pH (60 min) |
| F1 | 4.66±0.01 | 4.67±0.06 |
| F2 | 4.80±0.09 | 4.97±0.06 |
| F3 | 5.88±0.15 | 6.65±0.18 |
Rheology and viscosity
The rheological study of the three formulations of spironolactone suspension was carried out at a temperature of 25 ̊C. The results obtained for F1, F2, and F3 are shown in Table 3. F1 presented a low viscosity, but it tended to split into two phases. F2 and F3 remained homogeneous. F2 and F3 showed pseudoplastic behavior following the cross equation (r=1) (Figure 3). However, F1 presented a pseudoplastic but almost Newtonian flow.
Table 3 - Rheological behavior, viscosity, and thixotropy of the spironolactone suspension formulations.
| Formulation | Rheological behavior | Viscosity (mPa.s) | Thixotropy (Pa/s) |
| F1 | Pseudoplástic Quasi Newtoniano (ecuación de cross) | 48.69±0.21 | without thixotropy |
| F2 | Pseudoplastic (cross equation) | 72.02±0.31 | 47.49±0.34 |
| F3 | Pseudoplastic (cross equation) | 48.75±0.16 | 20.86±0.25 |
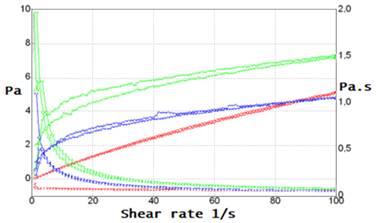
Fig. 3 - Rheological behavior of formulations F1, F2, and F3 (superimposed graphs) of spironolactone suspension 5mg/mL. The green and blue graphs show the pseudoplastic flow of F1, F2, and F3. The red colored graph shows the quasi-Newtonian flow of F1.
Particle size, polydispersion, and Z potential
Measurements were performed to determine particle size, polydispersion (PDI), and Z-potential at 25ºC of the three formulations of spironolactone suspension (n = 3). The results can be seen in Table 4.
Table 4 - Particle size, PDI, and Z-potential values of the spironolactone suspension formulations.
| Formulation | Particle size (µm) | PDI | Potential Z |
| F1 | 113.66±0.02 | 0.57±0.21 | -30.5±1.30 |
| F2 | 56.66±0.05 | 0.22±0.10 | -30.0±1.42 |
| F3 | 59.00±0.09 | 0.44±0.09 | -23.6±3.01 |
Of the three formulations of spironolactone suspension, F1 presented rheology more similar to liquids that are fluid and not very viscous, in addition to the fact that this suspension used to split into two phases and was the formulation with the largest particle size and PDI. On the contrary, F2 and F3 presented better results regarding rheology, particle size, and PDI being F2’s formulation of choice. This formulation presented the smallest particle size, PDI, and a good Z potential value (considering that a good Z potential value is between 30 and -30 MV).
Accelerated stability
To have a predictive criterion on the physical stability of the selected formulation (F2), a 10 mL sample of this suspension was taken and analyzed by DLS with the help of a TurbiscanTM Lab during 24 continuous hours of testing, obtaining the graph shown in Figure 4, where we will see the superimposed lines, which would indicate physical stability of at least six months. No precipitation, flocculation/coalescence, or rinsing phenomena were observed. The right and left ends of the graph indicate the empty part of the bottle.
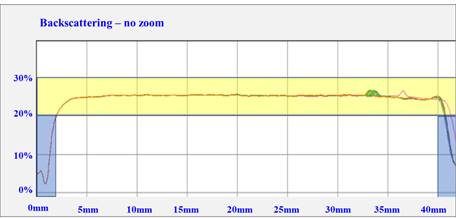
Fig.4 - Backscattering profile of the spironolactone F2 suspension corresponding to the 24-h assay. Yellow shows the overlapping lines (variations within the 10% allowed). Blue shows the lines generated by the apparatus when the laser beam passes through the empty part of the vial.
Quantification of spironolactone by HPLC
The amount of drug in F2 on the day of preparation was stored on days 15 and 30 at different temperatures: 4, 25, and 40°C. The results can be seen in Table 5. F2 degraded upon storage at 40°C as the days passed. At 4 and 25°C, the drug remained within the established parameters.
Table 5 Percentage of the drug in F2 for different days and stored at 4, 25, and 30°C.
| Day | percentage of drug quantified | ||
| 4°C | 25°C | 40°C | |
| 0 | NA | 95.50±0.02 | NA |
| 15 | 95.98±0.12 | 94.90±0.50 | 90.15±0.78 |
| 30 | 95.56±0.40 | 94.45±0.72 | 88.25±0.93 |
Dissolution test
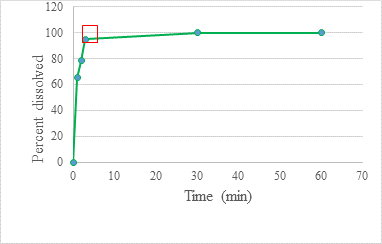
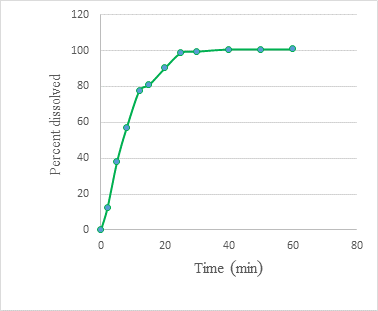
Dissolution profiles showed that the 5mg/mL spironolactone suspension (F2) could dissolve 99% of the drug in only 3 minutes, while the spironolactone tablets required 25 minutes to achieve the same percentage release as the suspension (Figures 5 and 6).
Microbiological analysis
In the microbiological analyses performed on F2, there was no growth of microorganisms in any of the culture media, as shown in Table 6. Likewise, no growth was observed in the plates for molds and yeasts. In the plates in which the spironolactone 5mg/mL suspension was used to isolate mesophilic aerobic microorganisms, total coliforms, molds, and yeasts, no growth of the microorganisms investigated were observed under any of the conditions tested. This was expressed in Table 6, reporting the absence of microorganisms in the plates containing the different culture media as <10 CFU/mL. This result satisfies the requirements established for non-sterile oral suspensión-type pharmaceutical products.
Table 6 - Microbiological analysis of F2 on different days and stored at 6°C, 40°C, and room temperature.
| Days | Suspension spironolactone 5mg/mL (Dilution 10-1) | ||||||||
| 6°C | 40°C | Ambient temperature (30±2°C) | |||||||
| VrBa | SMA | PDA | VrBa | SMA | PDA | VrBa | SMA | PDA | |
| 0 | N/A | N/A | N/A | N/A | N/A | N/A | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL |
| 15 | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL |
| 30 | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL |
Evaluation of the disinfection process of the container.
The swabbing performed on the primary container of the spironolactone suspension showed a negative result; there was no growth of the indicator bacillus (Bacillus subtilis). This meant that washing and disinfection of the bottles with 0.1% benzalkonium chloride was effective.
Discussion
Spironolactone is a synthetic steroid belonging to a class of potassium-sparing, aldosterone-antagonist diuretic drugs widely used to treat diseases in children (Shamma, 2016). The importance of making pediatric formulations lies in the lack of drugs containing adequate doses for these patients, considering that children are not small adults. Differences in physiology during development influence how drugs are absorbed, distributed, metabolized, and eliminated and cannot be predicted from adult data (Fernandez, 2011). Likewise, before being produced on a large scale or as a master formulation, a pharmaceutical formulation must have a series of previous studies whose results make us trust that the final product will be of quality as researchers and consumers. Quality raw materials play a vital role in the final product and its therapeutic action, which is why it is of utmost importance to study each of the components of the formulation (Provenza, 2014). In the present work, three formulations of spironolactone were carried out, and the formulation with the best results in terms of the studies performed, such as sedimentation time, pH, particle size, polydispersion, Z-potential, and rheological studies, was chosen. The other tests were performed only on the chosen formulation.
The procedure for the three formulations (F1, F2, and F3) was the same; the difference was basically in the choice of the suspending agent, in this case, CMC for F1 and xanthan gum in two different proportions for F2 and F3. The other products were chosen to obtain a pH at which the active principle would be more stable and biocompatible. Likewise, an antimicrobial was chosen to keep the product as stable as possible since microorganisms can affect the breakdown of chemical structures, destabilizing the formulations or contaminating them (Salgado, 2005).
The sedimentation time of F1 was 30 minutes, a situation that was not to be expected since the CMC used is considered a suspending agent par excellence, which is why a series of physicochemical studies were carried out to choose the best formulation. According to the literature, the pH at which Spironolactone is most stable is 4.5 (Gupta, 1991). In this study, the formulation with the most suitable pH was F1; however, Salgado et al. (2005) reported that pH does not affect the active principle in the formulations; however, these must be biocompatible. It was also observed that pH values could increase over time, probably due to the presence of potassium sorbate and other acidifying agents such as sorbic acid, which is why shelf stability studies at different temperatures are so critical and to observe pH changes over time (Salgado, 2005).
Of the three formulations, F2 showed a more monodisperse system than F1 and F3, in addition to having the best result in terms of its Z-potential value. These values are more relevant when compared to particle size and rheological behavior. In the literature consulted, it is recommended that the particle diameter in a suspension should not exceed 50 µm (Ervasti, 2020). In this study, 50% of F1, F2, and F3 showed an ideal particle size; however, the analysis of 100% of the same, if denoted measures above 100 µm, slightly exceeding this magnitude formulations, probably due to the sieve used in the preparation of suspensions. Due to this result, it was concluded that 60 minutes after reconstitution of the suspension and at rest, interactions probably occurred between the particles, causing aggregations of the same, much more noticeable when CMC was used as a suspending agent. In the case of xanthan gum, more excellent protection for these agglomerations was obtained, which evidences its capacity as a suspending agent. However, the subsequent studies related to the physical stability of the formulations provided more conclusive results. In the case of F1, the particles had a larger particle size, so they weighed more and sedimented more quickly, as was observed in the sedimentation time results (García, 2003). F2 had an ideal particle size and Z-potential effect, as formulations with Z-potential values between 30 and -30 MV indicate stable particles (Elmowafy, 2017). Although all formulations presented good Z-potential values, the smallest particle size and Polydispersion were precisely presented by F2.
Formulation F1 showed an unusual and unexpected rheological behavior since the suspending agent used was CMC, which should have presented a non-Newtonian flow and thixotropy, which did not occur; however, this result could have been caused by an expired raw material, poor storage, or changes in its crystalline structure, which could be the subject of study and submit this raw material to X-ray diffraction analysis. On the other hand, formulations F2 and F3 showed good rheological characteristics, F2 being the formulation that presented the highest viscosity and thixotropy; the behavior of these suspensions agrees with the study carried out by Alvarado M et al. (Alvarado, 2021). F2 showed adequate particle size, polydispersity, and Z-potential. Formulation F1 showed more common behavior in formulations of fluid characteristics such as solutions, syrups, and nanoemulsions (Provenza, 2014; Sosa, 2017). Given the above, formulation F1 was discarded, and F2 was chosen.
In at least 3-6 months, good physical stability of the F2 formulation can be predicted by observing the image obtained by multiple light scattering since no precipitation or coalescence was observed, and the superimposed graphs indicate that the sample is very stable. The particles are monodisperse at the temperature of 25 °C. A small peak is observed between 30 and 35 nm; this could be because suspensions are not homogeneous, as with syrups and other pharmaceutical forms.
On the other hand, quantifying the drug in formulations is essential to ensure its quality. The amount of spironolactone stored at different temperatures and days (0, 15, and 30) showed that the active principle is stable at 4 and 25°C even 30 days after preparation. It should be noted that this type of drug quantification study is "predictive," which gives an idea of how the formulation would behave when stability tests are performed with the temperature and relative humidity parameters stipulated in the standards of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH).
If the spironolactone suspension were to present results similar to those obtained in this study at the time of wanting to commercialize the formulation, the drug loss on day 30 stored at 40°C would be approximately 7.25% compared to the drug content obtained on the first day. This result would be considered significant according to ICH standards (a 5% variation from its initial value is considered a significant change). It is also recommended to quantify the drug by storing the formulation for extended periods, e.g., every three months during the first year, six months during the second year, and annually in the following years. It is essential not only to measure this parameter but also pH values, appearance, odor, color, and microbiological analyses should be performed in parallel (ICH, 2022).
Dissolution testing is a fundamental part of the quality control analysis used to evaluate dosage forms during development, stability, and quality control. Although we re-emphasize that this assay cannot be considered an official test, it does provide a rough favorable judgment on how well spironolactone is available from the developed formulation compared to the original product. To obtain better results, it would be considered appropriate to perform a study of the absorption of F2 using in vivo or ex vivo techniques, such as a study of drug absorption in the pig’s intestine using Franz cells. In this trial, it was observed that the release of the drug was faster from the suspension than from the Tablet; this is because the tablet requires the previous step of disintegration. This study’s results agree with other published studies (Provenza, 2014). spironolactone was released from the suspension in only 3 minutes, unlike the Tablet, which required 25 minutes.
Finally, F2 did not present microbial contamination; with the results obtained, it can be affirmed that the spironolactone suspension met the microbiological requirements established in USP 35 for non-sterile drugs. Likewise, adequate sterilization of the containers is essential. This study showed that using 0.1% benzalkonium chloride could sterilize the primary containers. Hence, a sterile container with a hermetic seal prevents formulations from being contaminated with microorganisms, regardless of the temperature at which they are stored (Bashan, 2014).
Conclusions
Three formulations of spironolactone 5 mg/mL suspension were tested, which differed by the suspending agent used and its concentration in the formulation. Suspension F2, where 150 mg of xanthan gum was added as a suspending agent, was the formulation that presented the best physicochemical characteristics and was selected to continue the study. According to the accelerated stability study, it was demonstrated that F2 would be physically stable for at least six months. As for the spironolactone, the content was stable for at least 30 days at two temperatures, 4 and 25°C. While at 40°C, the drug degraded, so it would not be convenient to store F2 at elevated temperatures. F2 could be used in temperate climate zones (zone I) and Mediterranean/subtropical zones (zone II). It could also be stored in environments where the temperature can be controlled (between 4 and 25°C) or in a refrigerator.
On the other hand, F2 released spironolactone in only 2 minutes, and no microbial contamination was observed during the evaluation period. Finally, it was demonstrated that sterilization with 0.1% benzalkonium chloride in the primary container did not allow the growth of microorganisms. F2 showed excellent values for sedimentation time, pH, viscosity, rheological behavior, particle size, polydispersion, and Z-potential, so we recommend future studies such as ex vivo absorption tests using pig intestine and in vivo effectiveness.