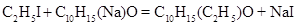INTRODUCTION
Life and career
Henry Baubigny was born in Paris on March 21, 1842, the son of Martial Baubigny and Adèle Pouchet. He took his basic education at the local schools and in 1859 received the diploma of bachelier ès sciences. He intended to continue his studies at the École Polytechnique but a sudden temporary illness did not allow him to take the entrance examinations. For this reason he enrolled at the Sorbonne where he studied under Jean-Baptiste André Dumas (1800-1884) and Victor-Joseph-Hippolyte Luynes (1828-1904) and in 1863 received his licence ès sciences. He then spent two years in Germany learning and doing research under the most famous chemists of that time (see below). In 1866 he returned to France and began his doctoral studies under Antoine-Jerôme Balard (1802-1876) and Paul Schützenberger (1829-1897) at the Collège de France. In 1869 he was awarded his degree of docteur ès sciences after successfully defending a thesis about camphor and its derivatives (Baubigny, 1869). The judges were Louis Joseph Troost (1825-1911), Paul Quentin Desains (1817-1885), and Henry Sainte-Claire Deville (1818-1881). In the introduction Baubigny mentioned that the first part of the thesis was done in the laboratory of Friedrich Wöhler (1800-1882) in Gottingen under the prompting of Rudolf Fittig (1835-1910), head of chemical projects; the second part was carried in Erlangen under the direction of Eugen Franz von Gorup-Besanez (1817-1878), and the thesis completed in the laboratory of Antoine-Jerôme Balard (1802-1876) and Paul Schützenberger (1829-1897), at the Collège de France (De Borniol, 1912; Lespieau, 1913; Anonymous, 2019).
In 1870 Baubigny married Marie Charlotte Goupil de Palières, a niece of Henry Sainte-Claire Deville (1818-1881).
Between 1869 and 1876 he worked at Établissement A. Poirier in Saint Denis (Seine), initially in the development and production dyes extracted from coal tar, and afterwards as head of production. In 1873 he received one of the three medals awarded to France by the Commission Spéciale du Jury des Arts Chimiques of the Exposition Universelle of Vienna for significant researches applied to industry. He then retired from the industrial activity and begun working in research in the laboratory of Deville at the École Normale Supérieure (1876-1897). In 1884 he was appointed répétiteur auxiliaire of chemistry at the École Polytechnique, after presenting a document describing his research achievements and publications (Baubigny, 1884c). In 1897 he was promoted to répetiteur adjoint and in 1899 to répetiteur titulaire (De Borniol, 1912 ; Lespieau, 1913 ; Anonymous, 2019).
Baubigny received several important awards for his scientific achievements, among them, Officier d'Académie (1891) and Officier de l'Instruction Publique (1897) (two appointments awarded to distinguished academics); and the 1901 Wilde Prize of the Académie des Sciences. He was also elected member of the Société Chimique de Paris and Chevalier of the Légion de Honneur (1908)
Baubigny passed away in Paris on October 15, 1912.
Scientific contribution
Baubigny wrote about 90 papers on the subjects of inorganic, organic and analytical chemistry, and natural products. In addition to the subjects described below, he synthesized some new salts of palladium (Baubigny, 1865-1866); studied the action of hydrogen sulfide on metallic salts (Baubigny, 1882a b c d); the synthesis of alabandine, a manganese sulfide (Baubigny, 1887a); the Schweitzer liquor and sky blue water (Baubigny, 1887b); the separation of cobalt and nickel (Baubigny, 1888) and of zinc and cobalt (Baubigny, 1889); kermesite (red antimony) (Baubigny, 1894b); proved that the vermillion of antimony was not an oxisulfide (Baubigny, 1894a); etc.
Camphor
In the introduction to his first project about camphor, Boutigny wrote that its general purpose was to study the reaction of sodium on ordinary camphor and the possible resulting derivatives, which he named compound camphors. There were two classes of these compounds, depending if the radical was oxygenated or not (Baubigny, 1866, 1868a).
The reaction between sodium and solid camphor did not seemed to be practical; it was better to use a solution of camphor in an inert solvent, preferable benzene or toluene, because they boiled above 900 to 110 0C. Careful heating of the mixture to 90 0C resulted in a copious evolution of hydrogen and disappearance of the sodium. The reaction was highly exothermic and for this reason had to be carried in a refrigerated flask provided with a condenser. The heating was stopped as soon as the reaction begun. Baubigny remarked that using one equivalent of camphor per one equivalent of sodium resulted in the disappearance of only 2/3 of the latter. The liquid on cooling gave crystals, which decomposed in contact with water or moist air, reproducing camphor. This instability made the crystals very difficult to purify and analyze. Baubigny assumed them to be sodium camphate, C10H15(Na)O, or camphor in which hydrogen was substituted by Na. Baubigny used sodium camphate to prepare a variety of derivatives (Baubigny, 1866, 1868a).
Non-oxygenated derivatives
Heating a mixture of sodium camphate to 600 to 70 0C in a water bath resulted in the reaction
with precipitation of sodium iodide. Water washes eliminated the sodium iodide and distillation the solvent of camphor The remaining mixture of unconverted camphor and the camphor ethylide (or ethylated camphor) could not be separated by solvents such as alcohol, ether, acetic acid, carbon disulfide, or chloroform because they dissolved both compounds. For separation purposes Baubigny used the fact that that both compounds had widely different melting points. The two phases were separated by filtration, accompanied by pressing the solid between fine papers to recuperate a much as possible of the accompanying liquid. All the liquid camphor ethylide was further purified by distillation. Baubigny described it as a very mobile and colorless liquid, having a burning taste, relative density 0.946 at 22 0C, insoluble in water, soluble in ether and alcohol, boiling without decomposition between 226 0Cand 231 0C (735 mmHg), not solidifying even when cooled to -20 0C, and having rotatory power [(]D = +61.4. Elemental analysis indicated that it contained, by weight, 79.66% carbon, 11.20% hydrogen, and 9.14 % oxygen. Baubigny indicated that this ethylide was the type of a first series of compounds in which one atom of hydrogen had been replaced by a methyl, ethyl, propyl group, etc. (Baubigny, 1866, 1868a).
Oxygenated derivatives
The next compound studied was acetylated camphor, belonging to a series where one atom of hydrogen had been replaced by an acetyl, benzoyl, and etc. group. The acetyl derivative could be obtained by reacting sodium camphate with acetyl chloride or acetyl bromide. Acetyl chloride was found not to react and for this reason Baubigny used instead acetic anhydride. A spontaneous lively reaction took place and the resulting acetyl camphor was separated by the same procedure used with camphor ethylate. Acetyl camphor was a colorless liquid, not solidifying even when cooled to -20 0C, boiling between 227 0C and 230 0C (733 mmHg), insoluble in water, soluble in oils, ether, and alcohol, with rotatory power [(]D = +7.5 and relative density 0.986 at 20 0C. Elemental analysis indicated that it contained, by weight, 73.95 % carbon, 9.85 % hydrogen, and 16.23 % oxygen (the slight excess of hydrogen indicated that some camphor was still present (Baubigny, 1866, 1868a). Baubigny also found that camphor dissolved in toluene was violently attacked by sodium at 90 °C and that the reaction could only be explained by assuming that sodium had replaced hydrogen in one portion of camphor and that nascent hydrogen combined with another portion of camphor, converting it into borneol, as illustrated by the reaction (Baubigny, 1868b):
C10H16O + Na = C10H15NaO + C10H17NaO
Reaction of camphor and borneol with alkyl iodides resulted in the substitution of one equivalent of hydrogen by the alkyl radical. The two homologues of ethylcamphor, methyl- and amylcamphor, were also described and shown to have properties similar to those of ethylcamphor. Methylcamphor boiled at about 194 0C (733 mmHg) and amylcamphor at about 277 0C (736 mmHg) (Baubigny, 1868b).
In 1868 Georg Malin reported that that he had reacted sodium (or potassium) and a steinöl (Tyrolean shale oil) solution of camphor for two hours and without controlling the temperature, and had noticed that the liquid became brown and eventually turned into a thick mixture from which he separated campholic acid. Malin remarked that during this process the alkali had not dissolved. Malin suggested that this acid was the result of the reaction (Malin, 1868):
C10H16O + C10H15OK = C10H17OOK + C10H14
Boutigny wrote that although he could not argue with Malin's result that the process had led to the formation of campholic acid, he could not accept that the reaction had taken place according to the above scheme. It was not possible that the metal alone was the source of the acid. To justify his assertion, he had boiled for many hours a solution of pure camphor with pure toluene in the presence of sodium or potassium, without obtaining campholic acid. In addition, it was known that KOH could be heated with camphor up to 300 oC, without reacting. Hence, it was hard to explain the role played by the compound C10H15OK over camphor in eliminating one molecule of water (Baubigny, 1868c). Consequently, Boutigny carried on two series of experiments. In the first one, he studied the action of sodium and potassium on dry and pure camphor dissolved in toluene pure that had been distilled over sodium. In every experiment of this nature he found that no boiling took place and only an extremely small amount of acid had formed. In the second series, he used two flasks. In one he introduced 10 g of camphor and dissolved it in enough anhydrous ether. Into this solution he added a sealed tube containing 1 g (and then 2 g) of sodium, and heated everything to 100 0C for 2 hours. Afterwards he broke the tube, cooled the contents, and then chased out the ether with steam. The flask was then sealed and the remaining material heated to 190 0C for 3 hours. Analysis of the product indicated that no cymene and no organic acid had formed but an equivalent amount of sodium borneol. All these results indicated that Malin's conclusions were wrong and campholic acid had formed for a reason different from that indicated in his equation (Baubigny, 1868c).
In a following publication Baubigny reported the action of several reagents upon borneol, camphor, and derivatives (Baubigny, 1868d e). For example, fuming nitric acid acted differently upon the ethyl and methyl derivatives of borneol and camphor (Baubigny, 1868d e). This acid rapidly dissolved and attacked the borneol derivatives, with release of heat and nitrous vapors; treatment of the product with water resulted in the regeneration of camphor. Nothing happened with the corresponding derivatives of camphor; they were insoluble in cold fuming nitric acid and dissolved only when heated to 100 0C; upon cooling, the solution split back into the two original liquid phases. Heating to a higher temperature transformed the mixture into a viscous mass of probably the nitro derivatives of the component, which were heavier than water. Borneol and camphor could also be differentiated by means of phosphorus pentachloride. The reagent attacked them already at room temperature, camphor slowly, yielding C10H15Cl, and borneol instantly, with release of heat and formation of C10H17Cl. At room temperature phosphorus pentachloride did not attack the alkyl derivatives but did at higher temperatures. When the mixture was heated to 100 0C in a closed tube, the pentachloride disappeared slowly and on cooling, no crystallization took place. Heating to a higher temperature resulted in charring and release of HCl (Baubigny, 1868d e).
The sodium derivatives of borneol and camphor were found to react with ethylene dibromide as follows:
C10H17NaO + C10H15NaO + (C2H4Br2)2 = C10H17O + C10H15O + 2NaBr + (C2H4Br)2
C10H15NaO + C10H17NaO + (C2H3Br)2 = C10H18O + C10H16O + 2NaBr + (C2H2)2
Baubigny found that the equimolar mixture of sodium borneol and sodium camphor reacted with CO2 to yield the compound C11H18O3, which he named camphocarbonic acid. This acid resulted from the simple addition of CO2 to camphor. Upon heating, it released CO2 and regenerated camphor (Baubigny, 1868d e).
All the above papers were the basis of Baubigny's doctoral thesis (Baubigny, 1869). In it, Baubigny mentioned that in spite of all the advances of chemistry the true composition of borneol still remained a subject of discussion. In 1859 Marcellin Berthelot had reported the synthesis of borneol and the history of camphor. He had found that at 180 0C the reaction of an alcoholic solution of KOH with two molecules of camphor resulted in the hydrogenation of one and oxidation of the second, yielding borneol and camphic acid (as predicted by Cannizzaro's reaction) (Berthelot, 1859):
2C20H16O2 + 2OH = C20H18O2 + C20H16O6 (using the old values of atomic masses)
camphor camphinate borneol
According to Berthelot, the fact that Théophile-Jules Pelouze (1807-1867) had previously conducted the opposite reaction and obtained ordinary camphor by oxidizing borneol with the chromic mixture (Pelouze, 1840):
3C10H18O + 2CrO3 = 3C10H16O + 3H2O + Cr2O3
proving that camphor was an aldehyde and borneol an alcohol. These assumptions had been reinforced by the failure of his experiments trying to esterify camphor with HCl, acetic and stearic acids.
Baubigny added that the purpose of his thesis was to see if it was possible to prepare mixed and composed esters of camphor in order to find its possible connection with alcohols (Baubigny, 1869). The first step in this direction was to find the possible action of alkaline metals on camphor. This idea was based on the known fact that alcohols reacted with these metals to form alkoxides (alcoholates), for example, ethanol reacted as follows:
C2H5OH + K = H +C2H5OK
The first experimental results indicated that it was possible to substitute one hydrogen equivalent of camphor by one equivalent of alkaline metal, sodium for example, with the help of moderate heat. The second observation was that the hydrogen liberated by the first reaction was not released but while nascent it totally recombined with camphor to form borneol, according to
2C10H16O + 2Na = C10H15NaO + C10H17NaO
These two intermediates were highly unstable and in the presence of water released borneol and camphor. Baubigny used the sodium magma to study their reaction with the iodides and bromides of mono and diatomic alcoholic radicals. In this manner he was able to prepare the methyl, ethyl, and amyl derivatives of camphor and borneol, which differed from the mother reagent by having one equivalent of hydrogen replaced by one equivalent of methyl, ethyl or amyl. For those that believed that camphor was an aldehyde, the product was a mixed ketone, while those of borneol were mixed ethers. These compounds had remarkable characteristics, as shown by their reaction with nitric acid and phosphorus pentachloride. In the case of diatomic alcoholic radicals the pertinent reagent was ethylene dibromide, which did not yield new products (the only products sodium bromide and acetylene). The same procedures were used with the radicals of mono and dibasic acids, for example, acetic acid and CO2. In the first case, Baubigny was able to isolate only acetoborneol, while in the second he found that CO2 combined directly, and in a simple manner, with camphor and borneol to yield C10H15CONaO2 and C10H17CONaO2. The salt of camphocarbonic acid was stable and crystalline, formed well-defined salts, and was decomposed by heat into camphor and CO2. The acid of the second compound was highly unstable; water decomposed its salts into bicarbonates and borneol (Baubigny, 1869).
The next section of the thesis gave a detailed description of the action of sodium on camphor, the action of water upon camphor sodate, the reaction of the sodates with the iodides of monoatomic alcoholic radicals, the bromides and iodides of diatoamic alcoholic radicals, the radicals of monobasic acids, and CO2. It also gave the formula and the chemical and physical properties of the numerous new products prepared (Baubigny, 1869).
At the end of his thesis, Baubigny devoted seven pages to analyse the vast experimental evidence he had accumulated and was forced to accept that it was not enough to decide to which functional category camphor belonged. He was sure it was not an alcohol; it seemed to be an aldehyde with too many exceptions, and speculated that it was probably a derivative of cymene and no more than that (Baubigny, 1869).
Transformation of amides into amines
Baubigny wrote than heating a primary or secondary amine, such as ethylamine and diethylamine, with a compound ester, such as methyl acetate, resulted in the formation of an amide, accompanied by the alcohol of the ester. Thus, ethylamine heated with methyl acetate yielded ethyl acetamide and free methanol. This amide could also be prepared in many cases, by heating the acid with a base (for example, acetic acid with aniline), or by simple distillation, like in the case of ammonium acetate. These amides could fix four volumes of water vapor and regenerate the primitive salt (Baubigny, 1882e).
In this communication Baubigny informed the Académie that he had found that these same amides were able to fix four volumes of alcohol vapor and regenerate the primitive salt or an amine where the amine had been replaced by a substituted amine, derived from the alcohol employed (Baubigny, 1882e). This reaction was totally general; it took place with methanol, ethanol, amyl alcohol, and also with aromatic alcohols such as benzyl alcohol. The acids acetic, valeric and benzoic had been observed. Baubigny also fond that to carry the fixation of the four volumes of alcohol vapor it was enough to heat to a temperature higher than the one needed for forming an amide. This finding led him to speculate if the same operation would produce the amide and the composite amine, by simple heating to a higher temperature. The results justified this assumption, thus, heating ammonium benzoate with ethanol, or aqueous ammonia with benzoic ester, yielded the same result as heating the benzamide with ethanol: benzamide was formed by elimination of water and then the ethylamine benzoate by the reaction of the alcohol for several hours at a higher temperature. The same phenomenon was observed then heating a mixture of aniline or phenylamine with glacial acetic acid and methanol: phenylacetamide and water were formed first, and then, by the action of the alcohol, methylamine and acetic acid
This generation process also indicated that the reaction could be repeated, so that together with the ethylamine benzoate and the remaining alcohol, a second step produced diethylamine benzoate, and a third, generated triethylamine benzoate. With aniline the reaction produced simultaneously, or in succession, acetanilide, methylaniline, methylacetanilide, and dimethylaniline. The ternary bases formed were unable to form amides. No ammonium derivatives were formed, a fact that distinguished this reaction from the procedure of forming composed amines by the reaction of alcoholic halides with amides. Clearly, the final product did not necessarily contain the tertiary amine or a mixture of different amines because it depended on the amount of alcohol employed and the duration of the heating process. It was to be expected that if it was possible to form amines from amides by the fixation of four volumes of alcohol, then it could also be expected that the cyanides derived from the amides would produce the same results by losing four molecules of water. Thus, four moles of alcohol would attach the corresponding amount of ethyl cyanide or ethylpropylaminde and yield eight moles of diethylamine propionate. Baubigny, 1882e. Baubigny also reported that heating acetamide with ethanol yielded ethylamine acetate, and heated with water it produced ammonium acetate. Similar results were obtained with methyl aniline or with aniline, or by heating acetanilide with methanol or water (Baubigny, 1886). In 1885 Robert Seifert had reported that alkoxides reacted with amides to yield amines substituted with the radical of the alcohol used (Seifert, 1885). For Baubigny, the use of an alkoxide was only a particular case of his procedure. For example, Seifert had written that the reaction:
C6H5NH(COCH3) + NaOC2H5 = C6H5NH(C2CH3) + CH3COONa
Took place when heating the mixture during 36 hours in a closed vessel to 1700-200 0C. If the mixture was slowly heated in an open vessel, then, upon distilling at 1600-170 0C passed a fraction of pure alcohol, almost identical to the theoretical amount, and leaving a residue of acetanilide sodate:
CH3CONH(C6H5) + NaOC2H5 = CH3CON(C6H5) Na + C2H6O
Hence ethylaniline was not the product of a double decomposition but of the following reaction between alcohol and acetanilide sodate (Baubigny, 1886).
Magenta
Charles Lauth (1836-1918) and Baubigny wrote that the dye aniline green (malachite green), was prepared by reacting salts of rosalinine (magenta, fuchsin, rosalinine hydrochloride) with an aldehyde, a hyposulfite, or alkaline sulfides. Years later, several chemists and industrial people realized that the intimate action of alcoholic iodides over rosalinine or violet of Paris resulted equally on the formation of a green, which was then named iodine green. Afterwards, it was found that a green could also be prepared by the dehydrogenation of dibenzyl aniline (Lauth and Baubigny, 1873). The high price of iodine led Lauth and Baubigny to test the possibility of replacing it in the many applications it had found in the dye industry. In this publication they wanted to inform the Académie of the results they had obtained in their factory A. Poirrier, in the manufacture of light green. Their process consisted essentially in the replacement of methyl iodide by a variety of other chemicals (i.e. ether, a mineral radical acid, sulfate, chlorhydrate, etc.). The preferred chemical was methyl nitrate in the presence of an alkali or an alkaline terreous. This communication served to establish their priority to the discovery (Lauth and Baubigny, 1873).
Equivalent of metals
According to Baubigny there had been many attempts to determine the value of the equivalent of metals through their sulfates; unfortunately, these had not been successful because of the difficult of obtaining the sulfates in neutral form and sufficiently pure. Successive crystallizations did not eliminate the last traces of free acid, some of these sulfates were decomposed by water; the process required heating and some sulfates decomposed at high temperatures. It seemed that the best process required heating the sulfates indefinitely at a constant temperature below the one of decomposition. Baubigny believed that these conditions could be fulfilled using a sulfur bottle, held at the temperature of boiling sulfur, 440 0C (Baubigny, 1883a).
Baubigny experiments showed this assumption to be true. With this bottle he was able to maintain a metallic sulfate at this temperature, higher than that of boiling sulfuric acid, without decomposition and for several days. Certain sulfates required much longer times to eliminate their free acid. This method allowed him to prepare, without difficulty, ferric sulfate, pure and neutral, as a white pink salt perfectly defined. The second phase of the process required the analysis of the salt. It involved a calcination process and weighing the boat and the salt before and after calcination. It was a dry process that involved only the handing of the boat. It did not carry all the errors inherent of an assay carried by the wet via. Clearly it was simpler than transforming the metal or its oxide into a sulfate, with all the associated weight losses (Baubigny, 1883a).
The dry sulfate was put in a platinum boat of known weight and the boat introduced into a tube having one end connected to a sulfur-stove. The whole was maintained at the stove temperature until two weightings, done at different times, were identical. This operation was carried by cooling the tube without contact with air and sealing it before removing it from the stove. Once the weight had reached a constant value, the boat was removed, put inside a platinum tube, and the whole, into a muffle. Heating was continued until the sulfate was completely decomposed. The boat was then cooled to dark red in the muffle and then removed and put back in the sulfur bottle to complete the cooling process (Baubigny, 1883a).
Baubigny used this method to determine the equivalent value of copper, zinc, nickel, aluminum, and chromium (Baubigny, 1883b c d, 1884a b). Cupric sulfate was prepared by dissolving the metal or artificial atacamite [CuCl + 3(CuO, OH)] in sulfuric acid. The equivalent value was found to be 31.698 (today's atomic mass 63.546) (Baubigny, 1883b). Zinc sulfate was prepared by dissolving distilled zinc in diluted sulfuric acid, evaporating to dryness and then heating to 440 0C. The equivalent value was found to be 32.667 (today's atomic mass 65.38) (Baubigny, 1883b). Nickel sulfate was prepared by dissolving commercial nickel nitrate in ammonia containing a small amount of carbonate, filtering, heating to eliminate the excess of ammonia, neutralizing with sulfuric acid, and separating the double sulfate formed. The salt was purified by successive crystallization and then transformed into sulfate by heating in an open muffle. The equivalent value was found to be 29.339 (today's atomic mass 58.69) (Baubigny, 1883c). Aluminum sulfate was prepared treating purified ammoniacal alum with a small excess of sulfuric acid. The equivalent value was found to be 13.508 (today's atomic mass 26.981) (Baubigny, 1883d). The equivalent value of chromium was calculated from pure chromium sesquioxide sulfate. This oxide was prepared by precipitation of sodium dichromate with hydrogen sulfide. The resulting chromium sesquioxide hydrate was then purified and dehydrated and converted into sulfate. The equivalent value was found to be 26.08 (today's atomic mass 51.996) (Baubigny, 1884b).
Efflorescence
Baubigny and Louis Victor Péchard (1862-1942) wrote that the pentahydrate of cupric sulfate was known to effloresce at atmospheric temperature and air humidity. They were interested in learning about the stability of this salt and for this reason they decided to study the efflorescence phenomenon in more detail (Baubigny and Péchard, 1892). Their first findings indicated that the phenomenon depended if the hydrate had been crystallized from a neutral or from an acid medium. This led them to extend their study to other simple or double metallic sulfates. A given solution of cupric sulfate was divided into two parts, one of them containing a small amount of sulfuric acid. The portions were then evaporated under vacuum, pressed under absorbing to paper to eliminate as much of the attached liquid, and the resulting crystals put in individual boats presenting more or less the same external surface. The boats were then located under a glass bell containing sulfuric acid as dehydrating medium. The samples were then removed and weighted at different times to determine the loss in weight. The experiments were carried in the temperature range 20 to 185 0C. The results indicated clearly that that the acid salt effloresced more rapidly than the neutral one. The first one was totally white when the second was just showing certain white points on its surface. Hence, if it was necessary to obtain a well-defined and stable hydrate it was indispensable that the crystals be rigorously neutral to methyl orange. This pentahydrate could be kept in dry air for a long time, without changing it composition (Baubigny and Péchard, 1892).
The experiments done with cobalt sulfate indicated that the stability of the heptahydrate in the temperature range 170 to 352 0C was not modified by the presence of acid and that the neutral hexahydrate was more stable than the acid one. The experiments with zinc sulfate in the temperature range 50 to 410 0C showed that the neutral sulfate and the acid one reached together a composition represented by the formula ZnSO4 + 2H2O; the acid salt lost rapidly three molecules of water while the neutral salt lost one. From that moment on the latter effloresced more rapidly, reaching the composition of the acid salt (Baubigny and Péchard, 1892).
The next experiments were done on the double sulfates of cobalt and potassium, zinc and potassium, ammonia alum, and chromium alum. These salts behaved differently from the simple sulfates; the starting solution had to be extremely acid in order to influence the efflorescence rate (Baubigny and Péchard, 1892).
Baubigny and Péchard published a more extensive and detailed memoir on the subject (Baubigny and Péchard, 1893). They mentioned that it was well known that the phenomenon depended only on the vapor pressure of water of the air surrounding the salt. Additional experiments showed that the amounts of water lost to dry air or dry CO2, were not influenced by the presence of sodium triphosphate or sodium carbonate; the development of the phenomenon was identical in both cases (Baubigny and Péchard, 1893).
Analysis and separation of halogens
Baubigny and Paul Rivals (1864-1939) wrote that the direct assay of chlorine, bromine, and iodine in a mixture of their salts had long be considered a very difficult task (Baubigny and Rivals, 1897a). The standard procedures employed indirect methods, which lacked accuracy and were hard to control. The procedure for assaying iodine in the presence of bromine and chlorine was the only one considered exact enough. These deficiencies had led them to look for better analytical methods. Their initial work was devoted to the assay of mixtures of chlorides with bromides and in their first publication they wanted to report the solution for two situations: (a) both halogens were present as alkaline salts, and (b) they were present as silver salts. Modern methods of separation of the halogens were based on the different behavior of their hydracids in the presence of oxidants such as lead dioxide, manganese dioxide, hydrogen peroxide, chromates, potassium permanganate, ammonium sulfate, etc., and small amounts of sulfuric or acetic acids. Léon Péan de Saint-Gilles (1832-1863) had shown that treating a neutral or alkaline solution of alkaline halides with an excess of potassium permanganate resulted in the oxidation of only and all the iodide in to iodate, without alteration of the chlorides or bromides, even at 100 0C (Péan de Saint-Gilles, 1858).
Baubigny and Rivals speculated that if potassium permanganate did not oxidize the bromides of potassium or sodium, perhaps it did oxidize other salts of the same halogens. Their initial findings indicated that permanganate did not attack a cold solution of cupric chloride but decomposed a solution of cupric bromide with liberation of the bromine. Hence, addition of neutral cupric sulfate would result in the formation of cupric chloride or cupric bromide. If the solution contained bromide, addition of a small amount of permanganate would result in the displacement of bromine. The next experiments using synthetic solutions of known composition, showed that these assumptions were correct and that the separation of the halogen was quantitative (Baubigny and Rivas, 1897a).
Baubigny and Rivas found that the decomposition of bromides by means of cupric sulfate and potassium permanganate did not allow the direct assay of bromine when the element was separated by diffusion under vacuum, because the reagents also attacked the grease of the connections (Baubigny and Rivas, 1897b). This obstacle led them to search for a mechanical procedure for separating the bromine, based on distillation by means of an air stream. Steam distillation was promptly discarded because of the huge amounts of water condensed together with the bromine. In addition, it led to a fast concentration of the cupric salt with the accompanying decomposition of the alkaline salts, which were stable at 100 0C. Using a stream of air solved the problem, with the additional advantage of being used at all temperatures (the entraining gas was now air instead of water). The passing alkaline liquid was first treated with SO2 (in order to reduce any possible compound of oxygenated bromine present), then with a solution of silver nitrate in nitric acid, and finally, brought to boiling. By these means any silver sulfide formed was converted into silver bromide (Baubigny and Rivas, 1897b).
In a following publication Baubigny and Rivas showed that the success of the procedure depended on using an appropriate excess of cupric sulfate and potassium permanganate. Thus a solution containing about 0.250 g of the alkaline salts should be added to 100 cm3 of another containing of 15-16% of CuSO4.5H2O and 7-8% of permanganate of potassium (Baubigny and Rivas, 1897c).
Baubigny and Rivals also studied the action of boric acid on iodides and its use for separating the iodine of iodides in the presence of bromides and chlorides (Baubigny and Rivals, 1903a). This procedure was based on the fact that pure boric acid was able to decompose, at room temperature, the iodides into hydrogen iodide, while it operated on saturated solutions of bromides and chlorides only when hot. The iodine was afterwards separated by the action of an oxidant such as manganese dioxide. The experimental apparatus and procedure were similar to the ones employed in the separation of bromine and chlorine (Baubigny and Rivals, 1897b). The solution tested was put in a round flask together with boric acid and manganese dioxide in predetermined amounts. Upon heating, the iodine volatilized and was carried out by means of an air stream into an alkali solution. The boric acid was used as a 10% solution in water. The manganese dioxide was used as a wet paste containing slight excess above the amount needed for the complete oxidation of the hydrogen iodide. Using larger amounts resulted in the partial oxidation of the iodine to iodic acid (Baubigny and Rivals, 1903a). In a following publication they reported that the oxidation of iodine to iodic acid could be avoided by adding cupric sulfate to the solution and separating the iodine as cuprous iodide (Baubigny and Rivals, 1903b).
Baubigny developed a very sensitive method for detecting the presence of very small amounts of bromine in marine salt, based to the fact that the halogen transformed fluorescein into eosin (a tetrabromide derivative) (Baubigny, 1897). He prepared the fluorescein by heating a mixture of phthalic acid and resorcinol for three hours at 1900 to 200 0C and then dissolving the product in a solution of 40-50% acetic acid, Writing paper was then immersed in the solution and then left to dry. The resulting fluorescein paper had a yellow tint, more or less intense depending on the concentration of the acetic solution. Addition of a small amount of a solution containing bromine colored the paper rose, typical of eosin, which contrasted with the color of the non-wetted section. According to Baubigny, this procedure allowed identifying the presence 0.001 g of an alkaline bromide in 5 to 10 g of marine salt (Baubigny, 1897).
Baubigny also looked for an accurate method for analyzing the mixtures of silver chloride, bromide and iodide (Baubigny, 1898). He found that the method he had developed with Rivas for soluble salts did not work in this situation. With a neutral solution, potassium permanganate showed no activity with silver halides, and the presence of cupric salts did not help. In his first set of experiments he tried to oxide these combinations in an acid medium, and operating all the time by the wet procedure. Using permanganate in the presence of nitric acid of density 1.34 showed that the iodide was oxidized to iodate and dissolved without loss of iodine. The bromide was also attacked in the cold, without loss of bromine. With chloride alone it was necessary to heat the mixture and the result was liberation of chlorine. Using potassium dichromate instead of permanganate did not improve the results; the iodide dissolved easily but the bromide and iodide did it very slowly, even at 100 0C. The chromo sulfuric mixture gave much better results; it acted slowly on the three halides at room temperature and very rapidly when heating in a water bath at 900 to 95 0C. The chlorine and bromine were released completely and the iodide oxidized to iodate, without traces of free iodine. The next stage was verifying that these processes were stoichiometric. For this purpose Baubigny applied the procedure to synthetic mixtures of known composition. A given amount of the tested mixture was put in a round flask, mixed with cold chromo sulfuric solution, and heated to 900 to 95 0C. It took no more than 20 to 25 minutes for the salts to dissolve completely. The gaseous chlorine and bromine were washed out by means of an air stream into an alkaline liquid contained in a condenser and precipitated with silver nitrate. The residue (containing the iodine) was dissolved in 300 to 350 cm3 of water, brought to boiling, then reduced by means of a stream of SO2 and finally, reprecipitated as silver iodide. The analysis of a synthetic mixture, containing, for example, 0.1357 g of silver iodide and 0.0382 g of silver chloride, gave 0.1354 g of silver iodide and 0.0382 g of silver chloride. Another containing 0.1357 g of silver iodide and 0.0408 g of silver bromide, gave 0.1354 g of silver iodide and 0.0404 g of silver bromide (Baubigny, 1898).
In a following publication Baubigny repeated his claim that with the help of the chromo mixture it was possible to separate quantitatively a wet mixture of silver iodide, chloride, and bromide. The method was equally precise if the precipitate was first dried for several hours at 1700 to 190 0C. The duration of the oxidation was longer when operating on the wet precipitate at room temperature (Baubigny, 1899a).
The next three papers dealt with the separation of bromides and chlorides when one of the two was in a much larger proportion than the other (Baubigny, 1899b c d).
Baubigny and G. Chavanne developed an analytical procedure for the assay of the halogen contained in organic compounds (Baubigny and Chavanne, 1903). The available methods were based on the oxidation of the compound by means of nitric acid, in the presence of silver nitrate and in a sealed tube (Carious' method), or on the destruction of the compound in a combustion tube, in the presence of calcium sulfate. In the Carious method, the weight of the insoluble silver salt produced reflected the amount of halogens present. In the second procedure, the contents of the tube were dissolved in diluted nitric acid, the carbon residue separated by filtration, and the solution precipitated with silver nitrate. According to Baubigny and Chavanne, it was time consuming and needed a high temperature (at least 200 0C) to assure complete oxidation. The procedure based on calcium sulfate was highly unreliable for compounds containing iodine. In addition, both methods did not give the content of each halogen, only the total of all the halogens present (Baubigny and Chavanne, 1903).
According to Baubigny and Chavanne, the procedure could be improved by employing the chrome mixture as oxidant. Upon heating, the chrome mixture burned vigorously the organic matter, the chlorine and bromine present were liberated completely and the iodine was totally retained by the solution in the state of iodic acid. The procedure was very rapid; it took a few minutes for a weight of 0.3 to 0.4 g of specimen. Baubigny and Chavanne gave a detailed description of the procedure to be followed for the case of iodine or chlorine and bromine (Baubigny and Chavanne, 1903, 1904).
Dithionates
In 1843 Pierre Berthier (1782-1861) reported the use of SO2 and sulfites in the assay of metals and ores (Berthier, 1843). He stated that silver sulfite in boiling water transformed into silver sulfate accompanied by a deposit of metallic silver; addition of an excess of an alkaline sulfite to the boiling water mixture resulted in the complete reduction of the sulfite. According to Baubigny, Berthier did not mention at all the formation of sulfuric acid, only the total separation of silver (Baubigny, 1909a, 1910b). Many chemists confirmed later this easy decomposition of silver sulfite in boiling water into sulfate, metallic silver, and SO2. Baubigny remarked that the process did not take place for dilute mixtures of sulfite because in this condition over 80% of silver sulfite decomposed into dithionate or hyposulfate, according to
2Ag2SO3 = Ag2S2O6 + 2Ag
justifying C. Geitner finding that the heating the solution to more than 200 0C resulted in the formation of large amounts of SO2 and SO3 originating from the decomposition of dithionic acid (Geitner, 1845). Baubigny mentioned that this fact had led many chemistry books to state wrongly that boiling a suspension of silver sulfite in water resulted in half the silver separating as metal and half dissolving as silver sulfate
2Ag2SO3 = Ag2SO4 + Ag + SO2
This incorrect statement had been extended to the double sulfites. According to Baubigny, heating a solution of a double sulfite to 100 0C resulted in the transformation of more than 95% of the salt into dithionate
2AgSO3Na = NaS2O6 + 2Ag
together the formation of a very small amount of SO2 (Baubigny, 1909a, 1910b). This reaction was analogue to the one reported by Walthère Victor Spring (1848-1911) for the double hyposulfite of alkalis and heavy metals (Pb, Ag, Hg) (Spring, 1874). In water at 100 0C they yielded a trithionate accompanied by the metal sulfide:
2 AgS-SO2-OK = (KSO3)2S + Ag2S
Baubigny made a series of experiments to calculate the yield of the reaction of decomposition of silver sulfite and found that 98.84% of it transformed into silver dithionate and only 10.16% into silver sulfate. In the case of double sulfites, the conversion varied between 95.96 and 96.4% (Baubigny, 1909a). He also found that light affected the sulfites (simple or double) in the same manner as heat, an outcome that explained the result reported by other chemists regarding the instability of cold silver sulfite in the presence of water: it remained white in the darkness and blackened slowly in the presence of diffuse light (Baubigny, 1909b, 1910b).
According to Baubigny the fact that the decomposition of silver sulfite and its double alkaline salts gave by the action of silver or light a neutral salt with loss of silver (half for the simple sulfite and total for the double salts), showed that the dithionic acid originated from the union of two sulfoxyl radicals, which could be separated by the action of sodium, and regenerating the sulfite
NaSO3-SO3Na + 2Na = 2Na2SO3
This result was another proof that the constitution of dithionic acid was HO3S-SO3H. Hence this acid was a disulfoxalic acid, which was related to sulfurous acid, HSO3H in the same manner that oxalic acid HOOC-COOH was related to formic acid HCOOH. This synthesis of dithionic acid provided a new argument in favor of the asymmetric formula of the sulfites, MSO2OM, proposed by William Odling (1829-1921) and other chemists (Baubigny, 1910a b).