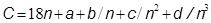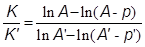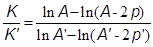INTRODUCTION
Life and career
Jean-Charles Galissard de Marignac was born in Geneva on April 24, 1817, the son of Jacob de Marignac, a judge and counselor of the State (Ador, 1902-1903). After finishing his basic education in Geneva, he entered the École Polytechnique in Paris, from where he graduated at the top of his class in 1835. He continued his studies at the École de Mines (1837-1839) where he was charged with scientific missions to Sweden, Norway, Denmark, and Germany. Afterwards (1840), he spent some months at the laboratories of Justus von Liebig (1803-1883) in Giessen, Germany, where he begun his research on the action of nitric acid upon naphthalene (Marignac, 1841a b c) and then at the Manufacture de Sèvres, Hauts-de-Seine, France; replacing Faustino Jovita Malaguti (1802-1878). In 1841 he was appointed to the chair of chemistry at the Académie de Genève, and then (1845) to the chair of mineralogy, a position he kept until 1878 when he resigned due to health reasons. In 1845 he married Marie Dominicé, five children were born of this union. Marignac passed away in April 15, 1894, after a long illness (Ador, 1902-1903).
Marignac received many honors for his academic and research activities. He was elected foreign associate of the Royal Society (1881), corresponding member of the French Académie des Sciences (1886), and honorary member of the Deutsche Chemische Gesellschaft (Chemical Society) of Berlin. He was awarded the degree doctor of sciences from the Universities of Heidelberg and Basel, was nominated chevalier of the Order of Sainte-Maurice-et-Lazare, and member of the Ordre Pour le Mérite für Wissenschaften und Künste (Civil order of Merit of Prussia (1888). In 1886, the Royal Society of London awarded him the Davy Medal (Ador, 1902-1903).
Scientific contribution
Marignac wrote more than 70 papers on the subjects of inorganic organic, mineral, and physical chemistry. In addition to the subjects described below, he also analyzed and described many minerals (Marignac, 1847, 1848); didymium and its combinations (Marignac, 1853a); crystallography (Marignac, 1856, 1858b); the isomorphism between fluosilicates and fluostannates (Marignac, 1858c); the atomic mass of silicon (Marignac, 1858c); tungstates (Marignac, 1863, 1864); the separation of the niobic and titanium acids (Marignac, 1868b); salts of beryllium and cerium (Marignac, 1873); saturation and supersaturation of solutions of calcium sulfate (Marignac, 1874a); discovered gadolinium and ytterbium, although he did not separate them (Marignac, 1878a b), etc.
Naphthalene derivatives
In 1835 Auguste Laurent (1807-1853) reported that the reaction between cold nitric acid and naphthalene generated two acids, nitronaphthalase, and nitronaphthalese acids, of formula C20H14N2O4 and C20H12N4O8, respectively (Laurent, 1835). Marignac found that longer times of reaction resulted in the formation of two additional products, one of which was an acid soluble in water and belonging to the same group as the two discovered by Laurent (Marignac, 1841a).
According to Marignac, boiling for a time naphthalene with nitric acid led eventually to the formation of Laurent's nitronaphtalese, which reacted feebly with nitric acid; upon distillation the nitric acid passed over almost entirely, with little formation of sparkling vapors. Afterwards, the temperature begun to increase and abundant red vapors were seen to form, which rapidly disappeared and were replaced by white vapors of subliming nitronaphtalese. These vapors decomposed suddenly accompanied by a weak explosion. The material remaining in the retort was a carbonaceous mass. Marignac added the nitric acid in small portions during several days until he noted that the reaction seemed to have reached an end. The resulting mass was separated by distillation into three different products: (1) an acid soluble in water, which Marignac named acid nitronaphthalic (nitrophthalic) acid; (2) an insoluble residue, which according to Laurent's nomenclature should be named nitronaphthalise (trinitronaphtalene?) and (3) a crystalline substances that sublimed and deposited in the neck of the retort and was soluble in nitric acid. Chemical analysis indicated that is was nitronaphthalese (Marignac, 1841a).
Marignac described nitronaphthalise as a fragile sulfur-yellow substance, melting at 100 0C, sparingly soluble in boiling ether, in boiling alcohol, and in cold water. It was soluble in aqueous alkalis and alkaline carbonates, forming a beautiful red solution, which afterwards turned black. Heated carefully it volatilized without leaving residue, but otherwise it decomposed suddenly with a weak explosion and a red flame. Elemental analysis indicated that it contained, by weight, 45.83% carbon, 1.91% hydrogen, 16.59% nitrogen, and 35.67% oxygen, corresponding to the formula C20H10N6O12 (Marignac, 1841a).
Nitronaphthalic acid contained, by weight, 45.73% carbon, 2.55% hydrogen, 6.59% nitrogen, and 45.13% oxygen, corresponding to the formula C16H10N2O12. It was completely insoluble in water and very soluble in ammonia; the boiling ammonia solution left to cool deposited colorless pearly crystals similar to those of naphthalene. Marignac prepared and described the properties of the nitronaphthalates of silver, lead, and barium (Marignac, 1841a).
In a following paper Marignac described the preparation of chloronaphthalese (tetrachloronaphthalene) hydrochloride, according the procedure described by Laurent (Marignac, 1841b). He passed a stream of chlorine through naphthalene, heating sporadically to melt the product and renew the surface. The resulting product had a butter consistency, melting easily in boiling water. It was washed several times with cold ether, which separated an oily green liquid and left the hydrochloride as a white crystalline powder. Chemical analysis indicated that it had the same chemical composition as the product prepared by Laurent; C20H16Cl8. Marignac boiled it with concentrated nitric acid and observed the release of abundant nitrous vapors. He believed that his product was naphthalic (phthalic) acid, although its composition differed substantially from the one reported by Laurent: It contained nitrogen and its silver salt contained eight atoms of carbon per atom of base. Marignac also reported the simultaneous appearance of a small amount of an oily liquid, colorless and transparent, having a very irritant smell and relative density 1.685 at 15.oC. It was insoluble in water, aqueous HCl, and nitric acid, and very soluble in ether and alcohol. The insolubility in water allowed its separation by steam distillation. Elemental analysis indicated that it contained, by weight, 7.08% carbon, 40.87% chlorine, 16.39% nitrogen, and 35.66% oxygen (Marignac, 1841b).
In 1841 Laurent reported the synthesis of a large number of new derivatives of naphthalene and his belief that the composition he had previously reported for naphthalic acid was erroneous (Laurent, 1841). Marignac wrote that Laurent's new composition was identical with the one he had reported previously and went on the describe the synthesis of hydrated naphthalic acid, mono ammonium naphthalate, silver naphthalate, and anhydrous naphthalic acid, as well as the reaction of ammonia with anhydrous naphthalic acid (Marignac, 1841c).
Hydrated naphthalic acid was prepared by treating chloronaphthalese with nitric acid; the resulting naphthalic acid was separated by repeated crystallization. The crystals were then dissolved in boiling water, on cooling the hydrated naphthalic acid precipitated as hexagonal or quadrangular thin plates. Elemental analysis indicated that their composition corresponded to the formula C16H12O8 = C16H8O6 + 2H2O. The mono ammonium naphthalate was obtained by slow evaporation of a neutral solution of the naphthalic acid in ammonia, the silver naphthalate by precipitating a boiling solution of ammonium naphthalate with silver nitrate, and the anhydrous naphthalic acid by sublimation of the hydrated form (Marignac, 1841c).
Marignac reported that hydrated naphthalic acid reacted with aqueous ammonia to yield ammonium naphthalate. According to Laurent, upon heating this salt decomposed to yield naphthalimide (phthalimide), a volatile compound. Marignac indicated that although anhydrous naphthalic acid was completely soluble in aqueous ammonia, the solution did not deposit ammonium naphthalate. The precipitated crystalline needles, redissolved in water yielding a slightly acid solution. Upon heating, these crystals lost water at about 100o to 120 oC and turned into naphthalimide, which was sparingly soluble in water; the pertinent solution was not acid.
Slow evaporation of this solution for a long temperature at a high temperature resulted in the precipitation of mono ammonium naphthalate. These properties led Marignac to assume that this particular substance was an amide, which he proposed naming naphthalamide (phthalamic acid) Elemental analysis of naphthalamide indicated that it contained, by weight, 61.29% carbon, 3.90% hydrogen, 9.15% nitrogen, and 25.66% oxygen, corresponding to the formula C16H12N2O5 (Marignac, 1841c).
Ozone
In 1843 Christian Friedrich Schönbein (1799-1868) reported the discovery of a new gas that originated during the electrolysis of acid water, and which he named ozone (Schönbein, 1843). This publication was followed by a number of others describing the properties and reactions of the new gas. In these Schönbein assumed progressively that ozone was composed of oxygen in a nascent state, afterwards, that it was a combination of hydrogen, oxygen, and nitrogen. This led him to assume that nitrogen was not an element but a gas that originated from the decomposition of ozone. He even assumed that it was a compound of hydrogen and oxygen. All these speculations moved Marignac to conduct a series of experiments to try to determine the nature of ozone (Marignac, 1845). Marignac prepared ozone first by Schönbein procedure and then by flowing air over sticks of phosphorus contained in a long tube.
Marignac concluded as follows (Marignac, 1845): (a) the ozone produced during the decomposition of water acidulated with sulfuric acid in a cell, was independent of the presence of nitrogen. No matter what method he employed to obtain a liquid absolutely exempt of any nitrogen compound, and all the precautions taken to dearate completely the water, its decomposition always produced ozone. The only precaution to be taken was to operate at a low temperature, because Auguste de la Rive (De la Rive, 1844) had proven that the ozone smell disappeared when the water was heated; (b) boiling lead dioxide with a diluted solution of sulfuric acid produced all the phenomena described by Schönbein, except that the smell that he had attributed to ozone seemed more to be that of nitrous acid, as shown by the fact that the gas produced turned litmus paper red and did not bleach it. No odor was produced when boiling a mixture of sulfuric acid with potassium dichromate; (c) the best method for producing ozone was to run a stream of air through a tube 1 meter long and 6 mm diameter, containing sticks of phosphorus; dry air did not produced ozone, the phosphorus became covered by a white layer, probably of phosphorus pentoxide. The emerging gas did not react with starch mixed with potassium oxide; (d) air, completely deoxygenated by passing through copper heated red, did not produce ozone with phosphorus; (e) pure oxygen did not produce ozone in contact with phosphorus; the resulting gas did not smell like ozone and did not react with starch mixed with potassium iodide; (f) nitrogen generated by boiling potassium nitrite with ammonium chloride did not produce ozone. An artificial mixture of one part of oxygen and four of nitrogen produced ozone in the same manner as atmospheric air; (g) addition of a very small amount of nitrous acid to air blocked completely the production of ozone; the nitrous acid turned litmus paper blue but did not bleach it; (h) carbon dioxide in contact withe phosphorus did not generate ozone; nevertheless, a mixture of 1 part of pure oxygen with 3 or 4 of CO2 produced ozone, in the same manner that a mixture of oxygen and nitrogen, although in less abundant amount; (i) hydrogen in contact with phosphorus did not produce ozone but did when mixed with a small amount of oxygen; the resulting gas turned litmus paper blue and bleaches it; (j) ozonized air, humid or dry, lost it odor and special properties when streamed through a tube heated to 300o or 400 oC; (k) ozone did not seem to be absorbed or altered by water, concentrated sulfuric acid, ammonia, or baryte water; (l) ozone was easily absorbed by a solution of KI; the liquid became immediately yellow and part of the iodine was liberated. Once all the iodide had decomposed, the solution became colorless and the ozone smell reappeared; and (m) ozone was easily absorbed by metals but only in the presence of humidity; silver turned brown black Totally dry ozone did not react with silver, copper, or zinc (Marignac, 1845). According to Marignac these results where a clear proof that ozone was composed of pure oxygen or oxygen and hydrogen. More experimentation was needed to decide between these alternatives (Marignac, 1845). In 1867 Jacques Louis Soret (1827-1890) demonstrated that the density of ozone was 1.5 time that of oxygen so that the ozone molecule should be composed of three atoms of oxygen (Sorel, 1867).
Freezing/boiling of sulfuric acid
According to Marignac, since commercial fuming sulfuric acid (oleum) contained a very small amount of SO3 perhaps it was possible to enrich it at lower temperatures. It was common knowledge that the acid possessed a hydrate containing 0.5 equivalents of water that crystallized 0 oC and that the monohydrate was very difficult to freeze. He was surprised to find that he could freeze at about 0 oC a fuming sulfuric acid that hardly fumed and that these crystals remained as such at several degrees about 0 oC. This finding led him to investigate if the knowledge about oleum was based on old mistaken information (Marignac, 1853b).
The first thing he did was to develop an analytical method for determining the correct concentration of the sulfuric acid being used. For this purpose he used an acidimetric method based on neutralizing the acid with a titrating solution of sodium carbonate prepared from fused sodium bicarbonate. All the crystallization experiments were conduced in winter when the night temperature varied between -4o and -6 oC. Marignac observed that at these temperatures ordinary fuming acid (titer 610.5) transformed into a crystalline mass impregnated with liquid acid. He separated the solid phase with a funnel and let it drip for several hours. He repeated the procedure several times, each at a somewhat higher temperature, and collected the liquids. This procedure separated the original material into a liquid phase (titer between 602 and 599.7) and a crystallized one melting at 10.4 oC (titer 612.8). These results indicated that exposure of the little-fuming acid to cold concentrated the SO3 in the portion remaining liquid, while the crystals had exactly the same composition of the monohydrate of sulfuric acid (Marignac, 1853b).
In another series of experiments Marignac concentrated commercial fuming acid by prolonged boiling and then subjected it to the same crystallization procedure. This time he found that the crystalline phase melted at 10.3 oC (the same temperature as in the previous series of experiments) and had a titer of 614, while the titer of the liquid phase was 630.8. These results negated the common knowledge that sulfuric acid concentrated by extensive boiling achieved the concentrated state of sulfuric acid monohydrate. Marignac also wrote that his method allowed a simple procedure for producing concentrated fuming acid, although limited to the days of strong cold (Marignac, 1853b).
Marignac observed that although sulfuric acid monohydrate melted at 10.5 oC, it was able to keep it liquid at temperatures well below that level. Crystallizing of the sub-cooled liquid was not achieved by agitation but by seeding it with crystals melting at 10.5 oC. This resulted in immediate crystallization and increase of the temperature to 10.5 oC. The monohydrate did not fume at ordinary temperature, only when heated to near 40 oC. Marignac measured the density of the acid (relative to that of water at 0 oC) at several temperatures and reported the values 1.854 (0 oC), 1.842 (12 oC), and 1.830 (24 oC) (Marignac, 1853b).
Ammonium chloride
Marignac wrote that the composition of gaseous ammonium chloride had been subject of much discussion. Its vapors occupied double the volume required by the theory of equivalent volumes and it was not known if this irregularity was a result of an anomaly in the physical constitution of its vapor or its dissociation into the elements (Marignac, 1868a). In 1865 Henri Sainte-Claire Deville (1818-1881) proved that the dissociation of ammonium chloride was not complete and that it varied with the temperature (Sainte-Claire Deville, 1863). He introduced a stream of ammonia and another of HCl into a space heated to 350 oC and noted a sudden increase of the temperature to 394.5 oC. This result showed that ammonium chloride did not decompose at 394.5 oC but that its components combined at this temperature with release of heat. Saint-Claire Deville also found that at 390 oC the density of the vapors of ammonium chloride was 14.64, relative to air. If Avogadro's hypothesis were true, the observed number would give a molecular mass of 53.5/4, far different from the one corresponding to the formula NH4Cl (53.5/2 = 26.75). If ammonium chloride was totally dissociated into equal volumes of ammonia and HCl then the density of its vapors should be (36.5/2 +17/2)/2 = 53.5/4 = 13.37. In other words, the observed value of 14.64 implied that the salt was partially dissociated.
According to Marignac, if the volatilization of ammonium chloride was only a simple change of state, it should only require an amount of heat comparable to that needed to perform the same change in other compounds. If, on the contrary, it was accompanied by partial or total dissociation, it should require a far greater quantity of heat, little different from that required by the chemical combination of ammonia gas and HCl (Marignac, 1868a).
Marignac understood that this was a very difficult experiment to carry on. For this reason he chose an alternative approximation, rough but sufficient, for answering the question. His apparatus consisted of a massive cast-iron cylinder of 10 cm diameter and 12 cm length, provided with three cavities of 3 cm diameter and 9 cm depth, perforated symmetrically about the axis. One of them housed an air thermometer and the other two the substance to be volatilized. This substance was placed in the cavities of the cylinder the moment it attained a temperature between 440o and 500 °C (the paper carried a detailed description of the apparatus and its operation). In this way Marignac determined that the volatilization of 1 g of ammonium chloride required 706 thermal units. This amount of heat was substantially larger than the heat of vaporization of the compounds for which this quantity was known, and particularly close to that determined by Pierre Antoine Favre (1813-1880) and J. T. Silbermann (1806-1875) for the chemical combination of ammonia and HCl at atmospheric temperature (743.5 thermal units) (Favre and Silbermann, 1847). Marignac corroborated this conclusion by determining the latent heat of vaporization of fifteen additional substances (i.e. ether, sulfur dioxide, acetic acid, butyric acid, ethanol, methanol, water, tin tetrachloride, phosphorus trichloride, arsenic trichloride, arsenic trioxide, mercurous chloride, mercuric chloride, mercury, and sulfuric acid). Marignac concluded that all the above results suggested strongly that ammonium chloride dissociated strongly when volatilized (Marignac, 1868a).
Double decomposition of salts
The publication of Julius Thomsen's (1826-1909) thermochemical researches about the heat effect accompanying a large number of chemical reactions (Thomsen, 1869) led Marignac to communicate his results respect the thermal effects produced during the double decomposition of salts, and the effect of water upon them (Marignac, 1869). For this purpose he prepared solutions containing 100 g/L of the substance in the anhydrous state, or (in the case of acids and salts) containing only constitutional water. He then added successive amounts of water so as to obtain diluted solutions containing 1 gram of solute per 10, 20, 40, etc. cm3 of water. He designated the resulting solutions as 1/10, 1/20, 1/40, etc. solutions. The heat of dilution was determined by mixing each solution with an equal volume of water and assuming that the specific heat of the solution was the same as that of water at the same temperature. Marignac measured the heat of dilution for four different situations: (1) a pure substance; (2) mixing of two substances that do not decompose; (3) dilution of a binary solution of substances that do not decompose; and (4) mixing two substances giving place to a double decomposition. The solutes examined included a large number of inorganic and organic salts containing a variety of cations (i.e. potassium, sodium, lead, calcium, etc.) and anions (nitrate, sulfate, carbonate, chloride, acetate, etc.) as well as free acids (sulfuric, nitric, HCl, etc.). In all cases the results referred to the mixing of equal volumes of solution and pure water (Marignac, 1869).
Marignac reached the following conclusions: (a) The dilution of a dissolution of a single solute is an exothermic or endothermic phenomenon accompanied by a change in temperature not exceeding 0.2 °C for 1/10 solutions, diminishing rapidly with the degree of dilution and becoming less than 0.01o to 1/40 solution. The thermal effect may be neglected below this limit. This rule, however, is not general. For example, in the dilution of sulfuric acid, the rise of temperature increases the more the solutions are diluted; (b) The mixture of the solutions of two salts, which do not react, produces in general less alteration of temperature than the simple dilution of each of these solutions. The process is exothermic when mixing solutions of salts containing the same base, for example, sodium chloride with potassium chloride, potassium nitrate and nitric acid with the nitrates of sodium, calcium and lead. The mixing effect is endothermic for salts capable of forming a double salt, for example, sodium sulfate with the sulfates of copper or zinc; (c) The dilution of a dissolution containing two salts, which do not react, with an equal volume of water, generally produces a very slight thermal effect, which is nearly equal to the sum of the thermal effects produced by diluting the two salts separately, and decreases rapidly as the solutions are more diluted; and (d) The mixing of two saline solutions, or of a salt and an acid, capable of resulting in a double decomposition, without forming an insoluble product, is accompanied by a more or less considerable thermal effect. Marignac wrote that this process was the hardest to quantify because of the difficulty of separating it from the complicated effects resulting from the dilution of the solutions employed. He gave as an example the action of sulfuric acid on sodium nitrate and sodium chloride, and of HCl or nitric acid on sodium sulfate: on the one hand, the action of sulfuric acid on an equivalent quantity of sodium sulfate releases 218 units of heat for 1/10, and 539 units for 1/80-solutions, and on the other hand, in the action of nitric acid on sodium sulfate, there is an absorption of heat varying from 1,965 to 1,255 units, accordingly as 1/10 or 1/80-solutions are employed (the paper provides a detailed table of the results for the four concentrations 1/10, 1/20, 1/40, and 1/80) (Marignac, 1869).
Properties of liquids
In 1871 Marignac reported the results of his measurements of the specific heat, density, and expansion of the aqueous solutions of a number of inorganic and organic solutes, at different concentrations. He also provided a detailed description of the equipment and experimental procedure, as well as an estimation of the possible errors. The following solutes were studied: sulfuric acid, sodium sulfate, sodium bisulfate, HCl, sodium chloride, and sugar (Marignac, 1870).
Specific heat
For each solute a table gave the concentration of the solution (n), expressed as moles of water per mole of solute, the molecular mass of the solution (p), the specific heat of the solution per unit weight (c) and per mole (C), and the difference between the latter and the specific heat of water (C - 18n). All the results were correlated by the general formula:
Marignac also reported the values of the same parameters for the solutions of sulfur, phosphorus, bromine, and iodine in carbon disulfide.
Density and expansion
For each solute a table gave the weight of the liquid (P), the temperature (t), the volume (V), the coefficient of expansion (( = dV/dt), and the density (D) relative to water at 4 oC. The pertinent results were correlated by the general equations:
V = 1+ aot + bot2
1 1
0 0 2
D= + a2 t + b2 t
d = + a0t
The numerical values of the different parameters are provided for various values of n (Marignac, 1870).
In a following publication Marignac reported the values of specific heats c and C for a large number of aqueous solutions of halides (16), nitrates (14), sulfates (11), chromates (4), carbonates (2), acetates (11), oxalates (2), and phosphates, arseniates, pyrophosphates, and metaphosphates (6) (Marignac, 1876).
Simultaneous diffusion of salts
Marignac wrote that Thomas Graham (1805-1869) had been the only one to study the simultaneous diffusion of binary solution of salts that did not combine (having a common anion or cation) and double salts (Marignac, 1874b). Salts that did not combine diffused separately and independently of each other, with the less soluble salt experimenting a decrease in its diffusibility, a result that suggested the possibility of their partial separation, analogous to the process of separating unequal ly volatile substances by distillation. Mixtures of potassium bisulfate and potash alum (a double sulfate) decomposed when diffusing; the first one yielded potassium sulfate and the second, alumina and potassium sulfate. The same decomposition phenomenon was observed during the dif fusion of double salts, such as the double sulfate of magne sium and potassium; in this case the diffusion was the identical as when the two salts diffused separately.
The rate of diffusion was approximately proportional to the concentration of the original solution; it increased with rise in temperature and was almost identical for groups of chemi cally similar salts at equal absolute (not molecular) concen trations and different with different groups. Although liquid diffusion was similar to gaseous diffusion and vapor ization with dilute solutions, concentrated solu tions showed a departure from the ideal relationship, simi lar to that in gases approaching liquefaction under pressure (Graham, 1850).
Marignac mentioned that the study of the simultaneous diffusion of two salts capable of forming double salts was important for clearing the controversy regarding the existence or non-existence of such salts in solution (Marignac, 1874b). His results indicated that there seemed to be no difference with the process that took place with non-reacting salts. Double salts were born only at the time crystallization began to take place, not in solution. Marignac developed the following formula to determine the ratio between the coefficients of diffusivity (K) of the salts dissolved in a given solution: If A and A' are the quantities of the two solutes, having coefficients K and K', and p and p' the quantities that have diffused after time t, then
dp = K(A - p) dt
dp' = K'(A' - p')dt
Integrating both equations gives
In practice this equation did not represent the experimental results. The upper liquid layers where the diffusion was taking place decreased their density more rapidly than that of the following layers, invalidating a basic supposition in the development of the above equation (uniform density of the solution, at any moment). Marignac took this fact into account by assuming that the diffusion of each salt decreased not in the amount p of each salt diffused, but in double that amount, so that the correct ratio K/K' would be given by
This equation was found to describe well the experimental results: the ratio K/K' was independent of the duration of the experiment. Another important result was that there was no difference in operating with mixtures prepared with equal weights of the components and mixtures prepared with the components in their equivalent (molar) ratio (Marignac, 1874b).
Marignac provided diffusion data for a very large number of binary solutions containing salts having a common anion or cation, and double salts. The pertinent tables reported the relative concentration of the solutes, the duration time of the experiment, the amounts diffused, and the value of the relative diffusibility (Marignac, 1874b).
Atomic masses
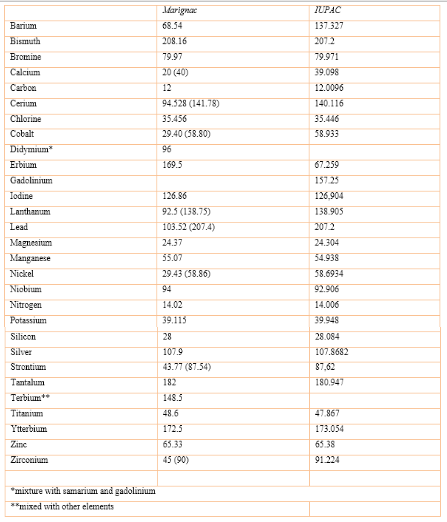
Marignac determined the atomic mass of 28 elements, expressed originally on the basis of equivalents, taking carbon as 12.5 and at a time when Prout's hypothesis was accepted by many chemists. Here we describe some of his procedures and results.
Chlorine, potassium, silver
Marignac wrote that after the work of Jean-Baptiste André Dumas (1800-1884) on the atomic masses of carbon and hydrogen, the attention of chemists had been directed to the possible validity of William Prout's (1785-1850) hypothesis that the value of the atomic mass of an element was an integer multiple that of hydrogen (Prout, 1815). Of all the simplest bodies, chlorine was the most important to know it's atomic mass accurately because it allowed easily to determine the mass of most known metals. Presently, the atomic mass of chlorine was assumed to be 442.65, a value that deviated clearly from Prout's hypothesis, which required it to be 437.5 or 450. This divergence led Marignac to conduct some work on the subject (Marignac, 1842).
Marignac based his experiments on the procedures used by Jöns Jacob Berzelius (1779-1848) to determine the atomic mass of chlorine: (1) fixing the equivalent of chlorine by determining the amount of oxygen lost by potassium chlorate when transformed into potassium chloride by calcination. Marignac described all the precautions he took to assure that the potassium chlorate was perfectly pure, to assure that the losses resulting from the entrainment of chlorate of chloride were negligible, and that the oxygen released was not contaminated with chlorine; (2) fixing the equivalent of silver nitrate by determining the weight of potassium chloride necessary to produce silver nitrate by double decomposition, and (3) analysis of the silver chloride to calculate the equivalents of chlorine, silver, and potassium (Marignac, 1842).
In 1845 Charles-Frédéric Gerhardt (1816-1856) wrote that in spite of the fact that most experiments pointed to the truth of Prout's hypothesis, the value of the atomic mass of chlorine continued to be a significant exception, and for this reason he had conducted his own determination of this property, using the experimental procedure of Marignac (Gerhardt, 1845). His experiments on the calcination of potassium chlorate yielded the same results of Marignac (expressed as the weight of residual potassium chloride). Nevertheless, Gerhardt mentioned that all the precautions taken by Marignac to avoid entrainment of potassium chloride by the emerging oxygen were not enough. For this reason Gerhardt added to the exit of the apparatus a U tube filled with wet cotton followed by another containing pieces of pumice stone humidified with sulfuric acid. The results indicated that this modification was enough to assure that no potassium chloride left the apparatus and, as a result, the real amounts of residual KCl were slightly larger than the ones corresponding to those of Marignac (on the average, 60.949 against 60.839). Calculation of the atomic mass of chlorine yielded exactly the figure 36, thus, justifying Prout's hypothesis (Gerhardt, 1845). Gerhardt's paper led to a polemic with Marignac centered on the possible experimental errors committed be each of them and on the validity of Prout's hypothesis (Marignac, 1846; Gerhardt, 1846).
Théophile-Jules Pelouze (1807-1867) suggested to Marignac a method for verifying the accuracy of the results he had obtained for the equivalents of chlorine, potassium, and silver. The procedure was based on weighing exactly quantities of silver and potassium chloride proportional to their equivalents, dissolving the silver in nitric acid, and then precipitating the silver nitrate with the potassium chloride, and verifying that the resulting filtrate did not become turbid upon addition of potassium chloride or silver nitrate. No formation of turbidity meant that the ratio of the equivalents was correct; otherwise it was easy to calculate the amount of silver or KCl necessary to add in order to obtain full precipitation (Marignac, 1843).
Careful execution of this procedure indicated that 100 parts of silver were precipitated by 69.098 parts of potassium chloride, an amount slightly different from the one obtained in the previous work (69.047) (Marignac, 1842). Although the difference was not large, Marignac decided to repeat his previous experiments taking special care to avoid the presence of KCl in his silver nitrate and loss of nitrogen oxide gases. This time he found that 100 parts of potassium chloride precipitated 192.269 parts of silver nitrate, instead of the 192.26 parts obtained previously. All these results led to correction of the previously reported values of the equivalents (silver, 1350; potassium, 490.01; and chlorine, 442.13) to silver, 1349.01; potassium, 488.94; and chlorine, 443.20). Assuming H = 1 the corresponding values of the atomic masses were: silver, 107.920; potassium, 39.115; and chlorine, 35.456 (Marignac, 1843).
Bromine, iodine, nitrogen, and calcium
Knowledge of the values of the equivalents of potassium and silver allowed an easy determination of the pertinent value for other elements (Marignac, 1843). Marignac used four procedures for determining the value of the equivalent of bromine: (1) determination of the amount of silver precipitated by potassium bromide; (2) analysis of silver bromide; (3) analysis of potassium bromate; and (4) analysis of silver bromate. Marignac reported that the first two methods yielded very accurate results, 999.60 and 999.30, respectively. The third method was very difficult to operate and did not allow working with large amounts of the reagent because of deflagration of the same; it yielded the value 999.98. The fourth method gave uncertain results due to the impossibility of drying completely the silver bromate, even when kept for long periods of time at 180 oC and under vacuum. The average of the three results was 999.63, which Marignac believed could be well rounded to 1000. On the basis of H = 1, the corresponding atomic mass of bromine was 80 (Marignac, 1843).
Marignac calculated the value of the equivalent of iodine by determining the amount of silver precipitated by KI and by the analysis of silver iodide. The use of potassium iodate was found to be unsatisfactory because it lost iodine during calcination. The first method yielded 1585.61 and the second, 1585.54, with an average of 1585.57. On the basis of H = 1, the corresponding atomic mass of iodine was 126.86 (Marignac, 1843).
The value of the equivalent of nitrogen was obtained by three methods: (1) analysis of silver nitrate; (2) precipitation of silver nitrate by potassium chloride; and (3) precipitation of silver by means of ammonium chloride. These methods yielded the values 175.07, 175.37, and 175.31, respectively (average 175.25). On the basis of H = 1, the corresponding atomic mass of nitrogen was 14.02 (Marignac, 1843). The value of the equivalent of calcium was obtained by analyzing calcium chloride, prepared by boiling limewater with HCl. This salt was dissolved in water, reprecipitated with ammonium chloride, and then evaporated to dryness and melted. The aqueous solution was then precipitated with silver nitrate. The pertinent value of the equivalent was calculated as 250, corresponding to one-half of atomic mass (40) (Marignac, 1843). All these results led Marignac away from Prout's hypothesis.
Barium, strontium, and lead
Marignac employed barium chloride to find the value of the equivalents of cerium, lanthanum, and didymium (Marignac, 1849a) and this led him to determine the value of the equivalent of barium, strontium, and lead (Marignac, 1858a). Many chemists had reported the value of the equivalent of barium, for example, Berzelius had calculated the value of this property as 68.43 (Berzelius, 1826). Pelouze had modified the procedure used by Berzelius and made it simpler and more accurate. His method was based on weighing approximately the equivalent weights of barium chloride and silver nitrate, followed by adding the barium chloride to a solution of the silver nitrate, and determining the amount of silver to be added to make the precipitation complete. Thus he found that 100 g of barium chloride precipitated 103.673 g, hence, the equivalent of barium was 68.67 (Pelouze, 1845).
Marignac repeated the experiences of Pelouze and found that 100 g of barium chloride precipitated 103.772 g of silver, meaning that the equivalent of barium was 68.57. Nevertheless, a careful inspection of Pelouze's method suggested the possibility that it was impossible to eliminate the water from barium chloride without losing part of the chlorine before the precipitation with silver nitrate. In other words, the reciprocal precipitation of silver and a soluble chloride did not rigorously corresponded to the equivalents of the two substances. To avoid this possible source of error Marignac proceeded to determine the value of the equivalent by comparing the weight of the silver nitrate employed in the precipitation of the barium chloride with the amount of barium sulfate produced, instead that of the barium chloride, thus avoiding the need to eliminate the water from the crystallized chloride. This time he obtained the value 68.58 for the equivalent of barium, corresponding to exactly one-half of the atomic mass of barium (137.16, with H = 1) (Marignac, 1858a).
A similar procedure was followed with strontium, using highly purified strontium chloride. The average result of all the experiences gave the value 43.77 for the equivalent of strontium, corresponding to one-half of the atomic mass of strontium (87.74 with H = 1) (Marignac, 1858c).
Marignac wrote that he had been unable to decompose completely lead chloride with sulfuric acid. For this reason, he opted for determining the weight of silver necessary for precipitating exactly a given weight of lead chloride dried at 200 oC, taking the necessary precautions to eliminate the action of light. He found that the value of the equivalent of lead was 103.52, corresponding to one-half the atomic mass of lead (207.1 with H = 1) (Marignac, 1858a).
The following table (Table 1) compares the value of the atomic mass calculated by Marignac with that published in Standard Atomic Weights 2013 (IUPAC Technical Report):