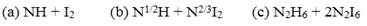Introduction
Life and career
The only information available about Amand Bineau (1812-1856) seems to be that contained in the eulogies pronounced by the geologist and metallurgist Joseph Fournet (1801-1869) and the zoologist and paleontologist Claude Jourdan (1803-1873) on the occasion of Bineau's death (Fournet, 1841; Jourdan, 1841).
Amand Bineau was born at Doue-la Fontaine (Maine-et-Loire) in 1812 He studied chemistry at the École Centrale des Arts et Manufactures and after graduation worked as head of the analytical laboratories of the École, taught chemistry at the École de la Martinière in Lyon, and worked as assistant of Louis-Jacques Thenard (1777-1857) and as préparateur of the chemistry course given by Jean Baptiste Dumas (1800-1884) at the Collège de France. In 1836 he received his degree of docteur ès-sciences after successfully defending two theses, one about the densities of vapors, the other about ammonium compounds (Bineau, 1837a b, 1838a). Shortly thereafter he replaced Victor Regnault (1810-1878) at the chair of chemistry at the Faculté des Sciences of Lyon. Year later he was elected Dean Faculty of Sciences, Lyon. Bineau also taught chemistry at the École de la Martinière in Lyon.
In 1839 Bineau was elected member of the Académie Impériale des Sciences, Belles-lettres et Arts de Lyon, and served as secretary of the chemistry class. He was also member of several important public committees of the city of Lyon, among them, the Commission des Soies, Commission Hydrométrique, and the Conseil d'Hygiène et Salubrité. In 1857 he was appointed chevalier of the Légion d'Honneur.
Bineau passed away in Lyon in 1856; his life was shortened by two serious accidents involving the leakage of chlorine and iodine vapors.
Scientific contribution
Bineau wrote about 35 papers, booklets, and books on the subjects of inorganic and organic chemistry, mineralogy, and botany. He edited the Leçons sur la Philosophie Chimique of Dumas (Dumas & Bineau, 1836) and collaborated in the publication of the last edition of Traité de Chimie of Thenard (Thenard, 1834-1836).
In addition to the subjects described below, Bineau also studied the composition of the hydrate of hydrogen iodide (Bineau, 1838; Estienne, 1838), the diformates of potassium and sodium (Bineau, 1847b), the compounds of camphor (Bineau, 1848a), the combinations of sulfuric acid and water (Bineau, 1848b, 1849), the chemical composition of rainwaters (Bineau, 1852), standard solutions for titrating ammonia (Bineau, 1853b) atmospheric ozone (Bineau, 1855b), etc.
In order to understand more clearly Bineau's publications, it is necessary to take into account that they were written at a time when hydrogen, the halogens, oxygen, and nitrogen, were assumed to be monatomic and the values of the atomic masses were different from the ones used today. All the chemical formulas that appear in this paper are written the way Bineau wrote them.
Density of vapors
As mentioned above, one of the doctoral theses of Bineau was devoted to the measurement and interpretation of the relative density of a large number of gases and vapors (Bineau, 1837b). He wrote that the relations that had been observed between the specific gravity of gases and their equivalent values had led many chemists to determine the relative densities of gases and vapors. Bineau measured the densities using the methods of Jean-Baptiste André Dumas (1800-1884) or Joseph-Louis Gay-Lussac (1778-1850). For a good description of these techniques, see Privat-Deschannel, (1873) for a detailed description of these techniques.
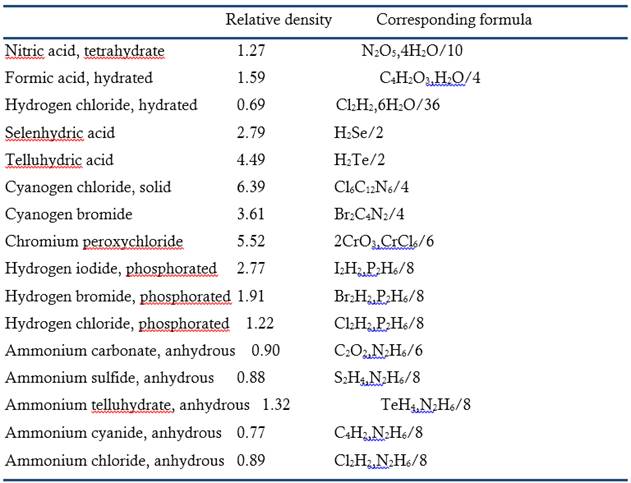
In his thesis Bineau reported the density and formula of the following gases:
For every, gas, he identified the procedure used, specified the numerical calculations, the pressure and temperature, and the number of equivalents corresponding to the formula.
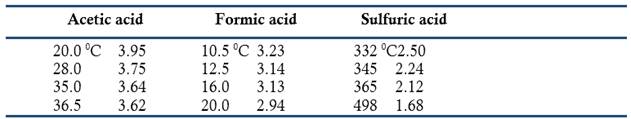
Bineau has also studied the phenomenon of abnormal density presented by gases such as acetic acid, formic acid, concentrated sulfuric acid, sulfur, phosphorus, and arsenic (Bineau, 1844b, 1846b, 1859). Charles Cagniard de la Tour (1777-1859) had reported this phenomenon in 1822 when studying the combined effect of temperature and pressure upon liquids such as water, alcohol, ethyl ether, and essence of petroleum, and attributed it to the molecules of the gas being at a closer distance than in a gas at low pressures (Cagniard de la Tour, 1822). Bineau gave a detailed description of the apparatus, experimental procedure, and equations used to calculate the correct density. For example, he reported that the relative densities of acetic, formic, and sulfuric acids vapors decreased with temperature as follows:
According to Bineau these, results showed that the influence of the distance between the molecules was negligible compared to the influence of the temperature. Not only that, justification of the abnormal densities using equivalent values required assuming that the molecules of the gas were not acting independently but as groups. In the case of acetic acid, the concordance between observed density and equivalents occurred only if the molecules were paired as a dibasic acid (dimer) (Bineau, 1846b).
Azeotropy
In a short notice published in 1842, Bineau reported that the ratio water/acid in almost all hydrated hydracids (HCl, HBr, HI, and HF) was larger that the one present in common acids, so that if one wanted to assimilate these compounds to salts where the water played the role of base, then it turned out that these would be extremely basic. Bineau had previously found that concentration of these acids by evaporation at room temperature left HCl associated with 12 equivalents of water, HBr and iodized HI with 9 equivalents, and HF with four equivalents. The latter results indicated that the causes that increased the tendency to vaporization were not proportional to the affinity of the acid for water. It seemed that during distillation there was a momentary split between the acid and the water, followed by the immediate recombination during condensation. In other words, the vapor produced was a simple mixture of steam and acid gas (Bineau, 1842).
These observations led Bineau to conduct a more detailed investigation of the phenomena that took place during the distillation of hydracids mixed with known amounts of water or halogen, in particular, one equivalent of HCl with 16 and 12 equivalents of water, one of HBr with 10 of water, HBr with bromine, and HI with iodine (Bineau, 1845c).
Bineau found that the mixture of one equivalent of HCl with 16 of water (20.17% wt. acid) had a relative density of 1.101 at 15 0C and, surprisingly, its boiling point varied with the nature and size of the vessel: at 750 mmHg it boiled at 107.5 0C in a platinum crucible and at 110 0C in a small flask of the same metal. The distillation in a necked glass retort occurred also at 110 0C. The volume of the vapor phase generated was equal to the sum of the volumes of the two components, when boiled separately. The relative density of the vapor, measured by Gay-Lussac's method, was 0.69 against the theoretical value 0.695. An interesting result was that HCl of this concentration changed little when in contact with the atmosphere, but in contact with dry air it became more concentrated. Also, kept inside a glass bell containing sulfuric acid and quicklime, the concentration of HCl increased to about 25% weight, corresponding to 1 equivalent of acid and 12 of water and the relative density to 1.128 at 14 0C.
Similar results were obtained with an aqueous solution of HBr; distillation of a very concentrated solution led to a decrease in the concentration of the acid. The opposite result occurred if the initial solution was diluted. In both cases the final concentration was about 46% acid, corresponding to 1 equivalent of water and 10 of acid, and relative density 1.486 at 20 0C. The density of the vapors was intermediate between that of the components, 0.975 against the theoretical value 0.974. Bineau wrote that a concentrated solution of HBr absorbed very large amounts of bromine (Bineau, 1845c).
Boiling an aqueous of HI, diluted or concentrated, always yielded a final solution containing 56.26% acid, corresponding to 1 equivalent of water and 11 of water, having relative density 1.70 at 15 0C, and boiling at 128 0C, at atmospheric pressure. The same procedure applied to iodated HI yielded a solution containing, by weight, 17.83% of HI (1 equivalent), 70.81% I (4 equivalents), and 11.36% of water (9 equivalents), and boiling at 142 0C at the pressure 750 mmHg (Bineau, 1845c).
In 1860 Henry Enfield Roscoe (1833-1915) and William Dittmar (1833-1892) measured the solubility of HCl and ammonia in water at different pressures and temperatures and showed that it do not behaved according to Henry's law (Roscoe & Dittmar, 1860). They also mentioned that Bineau had found that during boiling an aqueous solution of HCl at ordinary pressure the composition changed until it achieved the constant ratio of 1 equivalent of acid and 16 of water. If the same solution was exposed at ordinary temperatures to dry air it lost water until it attained the constant composition of one equivalent of acid and 12 of water. These results seemed to indicate that HCl formed two hydrates, which could be considered as chemical compounds. Roscoe and Dittmar wrote that is was also possible that "this composition was totally accidental and that it not implied the existence of any points of maximum attraction sufficient to justify the assumption of definite chemical composition". In order to test this assumption they distilled aqueous solutions of HCl at different pressures levels (0.05 to 2.5 atmospheres) and found that although each solution achieved a constant boiling point, this point occurred at different compositions, varying, by weight, from 23.2% HCl at 0.05 atm to 18.0% HCl at 2.5 atm. Hence, it was evident that no chemical compound was formed at this particular point (Roscoe & Dittmar, 1860).
In 1911 John Wade (1864-1912) and Richard William Merriman reported the variation of the boiling point of aqueous solutions of ethanol at pressures above and below the atmospheric pressure, a mixture known to boil at constant minimum temperature (78.15 0C) and composition (95.6% wt. alcohol) at normal atmospheric pressure (Wade & Merriman, 1910). In their paper they proposed that "in order to avoid the cumbrous periphrase mixtures having a minimum (or maximum) boiling point, to designate them as azeotropic mixtures ((, privative; ζέω, to boil). An azeotropic mixture resembles a chemical individual in boiling without undergoing change of composition, but differs from it in losing this fixed characteristic as soon as the pressure is altered" (Wade & Merriman, 1911).
Nitrogen iodide
Bineau wrote that the difficulty and dangers in handling nitrogen iodide had not allowed determining its exact composition and nature (Bineau, 1844a, 1845a b). Speculations about this question had suggested two possibilities: Jean-Jacques Colin (1784-1865) believed that the halide was composed only of nitrogen and iodine in the volumetric ratio 1:3 (NI3) (Colin, 1816), while Eugène Auguste Nicolas Millon (1812-1867) assumed that the compound was actually an amide iodide containing one volume of iodine, one volume of nitrogen, and two volumes of hydrogen, (NH2I) (Millon, 1838). Colin had discovered the iodide while studying the reaction of iodine and ammonia. His results indicated that dry ammonia reacted with dry iodine in two proportions, always yielding a liquid product, soluble in alcohol. The protoiodide was a red brown liquid having a strong ammoniacal odor while the deutoiodide was a viscous liquid having a metallic look. Both compounds were decomposed by water into ammonia iodide and nitrogen iodide. The latter was a black powder, spontaneously detonating when well dried, and insoluble in alcohol. The two ammoniacal derivatives and nitrogen iodide could be prepared simultaneously by adding an aqueous solution of ammonia to iodine, and separated easily with alcohol (Colin, 1816). Millon wrote that the reaction between water and nitrogen iodide resulted in the complete decomposition of the halide, the acids formed were saturated with ammonia and the precipitation of the iodine was accompanied by the release of nitrogen. These reactions were incompatible with the formula NI3 but well explained by formulas such as N3I3, or better, N2H4I2. The latter formula also justified the explosion by shock of the iodide, which was accompanied by the release of white dense vapors, probably of ammonium iodide, in the same manner that the explosion of nitrogen chloride, which was clearly accompanied by the release of white vapors of ammonium chloride. Millon added that the two liquids synthesized by Colin were probably a mixture of nitrogen iodide and ammonium iodide in an unknown proportion (Millon, 1838).
The analytical techniques used then were unable to select the correct formula because nitrogen iodide could not be analyzed directly. For these reasons Bineau used a different approach: instead of trying to determine separately the content of each element, he used different reactions to determine the ratio in which the elements were present (these are explained in detail in the paper published in 1845, including the preparation and properties of nitrogen chloride). Bineau wrote that the results of his experiences proved that both theoretical formulas were wrong and that actual composition of the compound corresponded to the formula NHI2 or N2H2I4, which could be written in the following alternative forms:
The first formula was the symbol of the theory that considered the detonating product a combination of iodine and a hypothetical compound, which Auguste Laurent (1807-1853) had denominated imide. Admitting that this compound was real or it was just an admission of the theoretical entity used to explain the composition of certain bodies, led to the compound being named hydrogenated nitrogen iodide or better, amide iodide. The second formula corresponded to the name ammoniacal nitrogen iodide; it represented a compound formed by two atoms of nitrogen iodide and one atom of ammonia, similar to other well accepted entities. The third formula corresponded to ammonia where two-thirds of the hydrogen had been replaced by an equivalent amount of iodine. According to Laurent, its name should be iodammoniaquèse (Bineau, 1844a, 1845a b).
Bineau concluded (1) that the names given to the iodine derivative of nitrogen, nitrogen iodide and amide iodide, were erroneous because it was actually composed of ammonia in which two-thirds of the hydrogen had been replaced by an equal quantity of iodine; (2) that the liquid resulting from the absorption of dry ammonia gas by iodine contained three equivalents of ammonia and two of iodine, and (3) that nitrogen chloride was formed of one volume of nitrogen and three of chlorine (Bineau, 1844a, 1845a b).
In 1905 Oswald Silverrad (1878-1960) proved that the correct formula of nitrogen iodide is NI3 and that one mole of the chloride is always accompanied by one mole of ammonia. The latter fact explains why previous researchers had found that the compound contains hydrogen (Silverrad, 1905).
Ammonia derivatives
Bineau wrote a doctoral thesis and several papers regarding the chemical combinations of ammonia and the role this compound played in chemical reactions (Bineau, 1837a b, 1839).
In the first paper he stated that ammonia was a remarkable compound, which held a middle position between the inorganic and organic bases (Bineau, 1837a). In chemical reactions, it was seen to play roles completely different, according if they were with acids or salts or in the presence or absence of water. As examples, Bineau discussed the preparation of ammonium iodide and the problems regarding its composition, the alleged reaction between phosphorus and ammonia, the reaction of ammonia with hydrogen sulfide, selenhydric (formed by one atom of hydrogen and one of selenium) and telluhydric acids, ammonium cyanide, ammonium iodocyanate, ammonium chlorocyanate, and ammonium bromocyanate. He also discussed the influence of heat on ammonium carbonate, sulfite and bisulfite, acetate, and benzoate, and the role performed by ammonia in reactions where it acted as a reducing (deoxidizing) agent. Quantitative analysis of the latter case required knowledge of the equivalent value of nitrogen (N2) (molecular mass or the quantity of nitrogen required for substituting 100 parts of oxygen); the usual value was 177.03, a figure that Bineau believed to be wrong. If nitrogen was the nitrure of hydrogen corresponding to water, then 177.03 of it were equivalent to 300, and its equivalent value should be one third of the accepted one, that is, 59.01. Bineau wrote that calculation of the equivalent value of nitrogen changed when considering substitution reactions of nitrogen alone (for example, cyanogen C4N2, and oxalic acid C4O3) substitutions done by hydrogen nitrides NH and N2H2 (for example, succinic acid C2H4O3 and succinamide C2H4O2NH, and oxalic acid C4O3 and oxamide C4O2, N2H4) (Bineau, 1837a).
Bineau wrote that the above facts indicated that (1) during the reciprocal decomposition of ammonia and an organic substance, the latter ceded to the hydrogen of ammonia one of its elements (usually oxygen) and received in place an equivalent amount of nitrogen or one of its groups with hydrogen, which acted as a simple body; (2) organic materials decomposed forming ammonia; (3) the equivalent value of nitrogen should be reduced to one-third of the commonly accepted value; (4) the hypothetical hydrogen nitrides NH and N2H4 should not be placed between the bases and acids, as suggested by August Laurent (1807-1853), but in the category of acidifiers; and (5) the group representing the equivalent of a radical should not be looked only as capable of keeping itself or of dividing, when new elements became attached to it (Bineau, 1837a).
In a following paper Bineau discussed the chlorides of cyanogene as a curious example of isomerism. Their analysis demonstrated not only the identity of their elemental composition, but also of the way in which they were formed and behaved (Bineau, 1838a). In both compounds it was considered that the carbon and nitrogen were united as cyanogen, and the role of the cyanogen was the same as that of simple bodies united to chlorine. Another interesting fact was that these isomers were frequently generated simultaneously, although the syntheses method offered no explanation for their difference. Bineau thought that a study of their decomposition by alkaline oxides could throw some light about this question. It could be assumed that the molecule of a solid chloride contained a much larger number of molecules than when present in the gaseous state; this assumption was justified by a comparison of the volatility of both chlorides and their density when in the gaseous state. Nevertheless, there was an apparent contradiction; in the one hand, the vapor density of cyanogen naturally solid was three times larger that of the gaseous chloride, indicating that a molecule in the first state contained three time more matter than a molecule in the second state; on the other hand, equal weights of their chlorides were obtained when decomposed by alkalis, one of them generated two equivalents of cyanuric acid and the other, three equivalents of cyanic acid. These results implied that the molecule of the solid chloride should weight only 1.5 times more than the molecules in the gaseous state. These considerations led Bineau to the study the following compounds they formed with ammonia:
Ammonium chlorocyanate
Bineau had previously given this name to the combination of ammonia with gaseous cyanogen chloride, which he represented by the formula ClC2N,C2H6. This formula indicated that in the presence of water the compound should decompose into ammonia chloride and neutral ammonia cyanides. However, this transformation did not take place, even under the influence of heat. Treatment of ammonium chlorocyanate with cold or boiling water not only gave a product not reacting like ammonium cyanate or urea, but upon evaporation it produced a white crystalline mass, which upon heated turned into a yellow mass, fixed and stable under a nascent red temperature. After heating to a higher temperature this mass became completely depleted of chlorine. Burned with cupric oxide it produced a very small amount of water and a gas composed of about 2/3 of CO2 and 1/3 of nitrogen. According to Bineau, these results meant that the residue was a carbon nitride of formula C3N2 (assuming C = 38.2), that is, the same substance that Justus von Liebig (1803-1883) had named mellon (Bineau, 1838a).
Ammonium chlorocyanate was not attacked by HCl; sulfuric acid dissolved it generating HCl exempt of CO2, and nitric acid attacked it slowly producing CO2, HCl, and a little of chlorine and nitrous vapor. Treatment with KOH decomposed it producing ammonia. The original compound turned litmus deep red (Bineau, 1838a).
Ammonium parachlorocyanate
Bineau gave this name to the combination obtained by simple contact of ammonia gas with finely divided cyanogen chloride, during 24 hours in the dark and at room temperature. The resulting product was found to contain by weight 73.1% cyanogen chloride and 26.9% ammonia, corresponding to 1 volume of gaseous cyanogen chloride and 4 volumes of ammonia. Bineau wrote that the properties of ammonium parachlorocyanate were very similar to those of chlorocyanate: It was a white odorless solid, without much taste, and stable at temperatures up to 1000 to 130 0C; at higher temperatures it decomposed without fusing, into HCl, ammonium chloride, and a white fusible and unstable matter, which decomposed into ammonia and left a residue having the properties of mellon. Ammonium chlorocyanate was sparingly soluble in water and the aqueous solution clouded silver nitrate and reddened the litmus tincture after a long contact. The compound was hardly attacked by aqueous HCl, but sulfuric acid dissolved immediately with disengagement of HCl pure. Nitric acid did not exert an immediate action but after some hours transformed the salt into an agglomeration of crystalline needles of cyanuric acid. Potassium hydroxide dissolved the ammonium parachlorocyanate, with release of ammonia (Bineau, 1838a).
Ammonium bromocyanates
Bineau found that cyanogen bromide and ammonia combined in two different proportions, yielding a liquid and a solid compound. The first one was a salt having an excess of the base; the second seemed to be neutral bromocyanate. Liquid bromocyanate was obtained by exposing cyanogen bromide to the action of ammonia; at the end of the reaction the crystals of cyanogen bromide turned into a colorless liquid, containing, by weight, 50.4% of cyanogen bromide and 49.6% of ammonia, corresponding to the 2 volumes and 12 of the latter. This combination was colorless and had a strong smell of ammonia. In contact with air it rapidly decomposed releasing most of its ammonia and transforming into colorless needle crystals of solid ammonium bromocyanate, containing, by weight, 75.3% cyanogen bromide and 24.7% ammonia, corresponding to 2 volumes of the first and 4 of the latter. This solid was odorless and had a very sharp taste like ammonium chlorocyanate. It was not affected by air but ammonia transformed it slowly into liquid ammonium bromocyanate (Bineau, 1838a). In another publication he reported that cyanogen bromide melted between 490 to 50 0C and boiled between 640 to 65 0C (Bineau, 1838b).
Bineau also reported the synthesis, composition, and properties of ammonium sulfide, ammonium cyanide, ammonium sulfoarsenite (As2S3,2NH3), and ammonium sulfophosphite (P2S3,2NH3) (Bineau, 1838a).
Ammonia and nitrogen
In several of the papers exposed above Bineau mentioned that he had developed a more accurate procedure for the quantitative determination of ammonia and nitrogen. In two papers published later he gave a detailed description of his method (Bineau, 1846a, 1851). The pertinent apparatus consisted of two parts: a small retort provided with a long neck, and a glass tube, 2 mm diameter, curved several times like a U tube, having two branches bent at an obtuse angle. One of the branches was shorter than the other. The shorter branch was connected to the neck of the retort, and the longer filled with glass fragments. The ammonia was determined by absorption in an acid solution (sulfuric or HCl) of known titer, located in the longest branch. The amount of acid used was in excess of the ammonia released. The sample to be examined was put in the retort, together with a small amount of calcium carbonate to decompose the ammonia combination. The bottom of the retort was heated with burning coal until boiling of the contents and the process continued till all the gas had been used. The acid solution was then titrated and a simple calculation allowed determining the amount of ammonia released from the sample (Bineau, 1846a, 1851).
Calcium carbonate
In a following publication, Bineau described a similar procedure for the dosage of the calcium carbonate contained in limestone, marls, arable lands, and waters; a subject extremely important for those working in the arts of construction, geology, and agriculture (Bineau, 1847a).
In the case of solid materials, the substance being examined was first finely divided and then introduced into a retort having a tubular neck, together with HCl titrated, in an amount in excess of the amount of carbonate. The neck was connected to a receiving vessel and then the mixture was brought to boiling until the reaction was complete. The liquids present in the retort and the receiver were mixed, a small amount of litmus added, and everything neutralized with a standard caustic solution (Bineau, 1847a).
Bineau indicated that the results of his method were not affected by the presence of aluminum or ferric oxides. In the case of other impurities, it was necessary to proceed as follows: (a) if the sample contained magnesium, it was first treated with enough aqueous solution of sugar (together with additional HCl titrated) until no more precipitate was formed. The amount of magnesium was determined from the difference in the volumes of alkali used in titrating the original solution and the filtrate of the second operation, as well as the amount of alkali that had displaced the magnesium; and (b) if the sample contained sodium or potassium carbonates, it was first washed with water to eliminate the soluble carbonates; the rest of the carbonates were eliminated by precipitation with ammonium oxalate. Manganese dioxide was the only material that could destroy HCl by means different from neutralization. Bineau added that the same analytical procedure could be used replacing the HCl by nitric or sulfuric acids, except that the sample had to be divided more to insure that the acid achieved the complete destruction of the carbonate (Bineau, 1847a).
Carbon dioxide and carbon
According to Bineau, the dosage of gaseous CO2 was particularly easy: the gas was reacted with a measured (and in excess) volume of standard caustic hydroxide (preferable KOH), followed by addition of a neutral barium salt and agitation. The filtrate (or part of it) was then titrated to determine the amount of free alkali. If the sample tested did not contain other impurities, the barium salt could be replaced by a solution of calcium sulfate. It was important that the water used in all these operations be free of CO2 (Bineau, 1853a).
Magnesium
In another publication Bineau extended the details regarding the analysis of calcium carbonate in the presence of magnesium. He indicated that if the magnesium was present in small amounts, say two to three percent, it caused an error in the determination of the calcium of only 0.5 to 0.75%; a very small (and acceptable) error in a typical analysis of calcium. Bineau wrote that especial care had to be taken to the presence of CO2 in the water because it increased significantly the solubility of barium and magnesium carbonates (Bineau, 1858a).
All the papers describing the above methods included detailed numerical examples of the calculations to be carried for reaching the appropriate result.
Solubility of carbonates and oxides
Bineau wrote several papers reporting the solubility in water and properties of the dissolution of metallic carbonates and oxides (Bineau, 1855a, 1857a b). The experimental techniques, procedures, and results are discussed detail in an extensive report published in 1857 (Bineau, 1857b). Bineau warned that commercial litmus was not sufficiently sensitive; previous to use it had to be completely deprived of the alkaline carbonates that accompanied it. Boiling it with a slight excess of a strong acid and then neutralizing the remaining acid with calcium carbonate easily achieved this.
Bineau's main conclusions were as follows:
Calcium carbonate. It dissolves in water in the ratio 1/50,000, a value that does not change between 18 0C to 100 0C. The carbonate of lime furnished nearly the same indications of solubility, both with cold and hot pure water, or in common water of which the natural bicarbonate had been destroyed by a long continued boiling. Its solubility is substantially increased by CO2 dissolved in the water.
Strontium and barium carbonates. Water dissolves about three hundred thousandths of the first and four of the second.
Magnesium carbonates. Its solubility in water, one or two hundred thousandths of the weight of water, is less than that of silver oxide, lead monoxide and mercury (II) dioxide; but the result is powerfully modified by the presence of CO2. Magnesium bicarbonate, after sufficient washings, ultimately dissolves in the proportions of only about 0.l g per liter of water (or 1/10,000), whether cold or hot. The solubility is substantially higher in the presence of CO2. The aqueous solution of bicarbonate of magnesia presents most of the reactions of potassium and sodium carbonates.
Potassium hydroxide. Bineau reported the water solubility of KOH and the relative density of the pertinent solutions, between 00 (66.7 g/100 g water and 1.51) and 57 0C (100 g/100 g of water and 1.61).
Sodium hydroxide. The crystals of NaOH are little efflorescent in dry air and extremely efflorescent in humid air. Their solution has a great aptitude to become supersaturated. Bineau reported the water solubility of NaOH and the relative density of the pertinent solutions, between 00 (58.5 g/100 g water and 1.52) and 64 0C (96 g/100 g of water and 1.63).
Mercury(II) oxide. Prepared either by the dry way or by the humid way it dissolves in from 20,000 to 30,000 times its weight of water. Its aqueous solution has no action on purified litmus, and water tinged with the reagent, and then slightly acidulated retains its tint after having been charged with mercury(II) oxide; a small quantity of saline water manifests in it an intense alkaline reaction.
Silver oxide. Water dissolves about l-3,000th of its weight of silver oxide. This solution of produces a double decomposition not only with the haloid salts, but also with the phosphates.
Lead monoxide. Litharge seems to be insoluble in water but this is not the case of the oxide prepared by the humid way. One part of this oxide form dissolves in 7,000 parts of water; this dissolution is capable, like silver, of decomposing haloid salts and oxysalts, such as the phosphates, chromates, oxalates, carbonates, sulfates, and nitrates, with liberation of alkaline oxide. In all the cases the displacement of one equivalent of alkali requires several or at least 1.5 equivalents of lead monoxide. The easy production of caustic alkalis when their salts are reacted with lead oxide explains partly the preservative effect that the alkaline compounds exert on this metal.
Zinc oxide. According to Bineau, the solubility is also dependent on the mode of production. It dissolves about one part in millionth water; the solution is sensible however, to purified litmus.
Ferrous oxide. Its solution, which was obtained by means of iron and pure water slightly aerated, contained about l-150,000th. It had a very strong ferruginous taste; it became turbid, ferric oxide being formed, as soon as it came in contact with the air; before oxidation it exerts an alkaline reaction on neutral or slightly acidulated litmus.
Alkali oxides. Their solubility in water is as follows: 1/750 for chalk at 18 °C; 1/1500 at 100 °C; 1/130 for strontia at 20 °C.; 1/29 for baryta; 2/3 for sodium; and 1 for potassium.
Other oxides included magnesium aluminum, chromium, manganese, tin, antimony, and copper (Bineau, 1857b).
Ammonia absorption by algae
Bineau was led into this subject after observing the growth of microorganisms growing in waters containing residues of ammonia (Bineau, 1856a). He studied in particular the behavior of the two algae Hydrodiction pentagonale and Conferva vulgaris, the first having a reticular texture and the second long deep green filaments. For this purpose he placed amounts approximately equal of both specimens in closed bottles holding 250 cm3 of an aqueous solution containing 12 ppm of ammonia (as ammonium chloride), and a little less of calcium nitrate. One of these bottles was put in a window exposed for 11 hours to sunlight, and another nearby in the dark so that both would achieve, more or less, the same temperature. After 10 days the liquids were filtered and analyzed for ammonia content. The results indicated that the Conferva and the Hydrodiction algae samples exposed to sunlight had absorbed about 75% and 50% of the ammonia original present, and only about 50% of these amounts when kept in the dark. In all cases the nitrate had disappeared completely.
Bineau conducted a second set of experiments, this time keeping the algae in an aqueous solution containing 53 ppm of ammonium nitrate. One sample was kept in the dark, another in diffuse light, and the third exposed to direct sunlight. Once again, the presence of light led to an increase in the amount of nitrogen absorbed (Bineau, 1856a).
Bineau reached the following conclusions: (a) algae seem to be able of absorbing or decomposing ammoniacal salts with intensity analogous to that of CO2. This has hitherto had no parallel in the case of saline matters, which were generally absorbed much less abundantly than their solvents; (b) the aptitude of algae to able to consume the nitrates present in their growing aqueous habitat, be by the direct absorption of the nitrogen contained in these salts, or by their conversion into ammonium salts; (b) the apparent efficacy of sunlight for facilitating the elaboration of nitrogen compounds by green vegetables, as well as that of CO2 (Bineau, 1856a).