Introduction
Morinda royoc L. (Rubiaceae), commonly known in Cuba as garañón, piñipiñi or piña ratón arbusto, is a plant typically found in coastal hammocks, and rarely inland (Roig y Mesa, 1988). The plant is a vine sprawling shrub with large bright green elliptical leaves and small white flowers throughout the year. The fruit results from coalescence of the inferior ovaries of many closely packed flowers, it has a smooth surface and many polygonal sections. The immature fruit is green. As it matures, the fruit becomes yellower in color, and unless it is harvested at this stage, it simply falls to the ground. As it ripens, the fruit change to a dark brown color with a very pungent smell, like the odor of rancid cheese and its taste is sour. In Cuba, the roots are used to make a product with stimulating, revitalizing and anti-stress activity that also increases the libido (Scull et al., 2000).
Although the fruit of noni (Morinda citrifolia L.), a related species used as folk remedy, has been broadly studied (Chan-Blanco et al., 2006; Potterat & Hamburger, 2007; Pino et al., 2009, 2010a, 2010b), very few reports on the chemical composition of M. royoc are available, particularly related to the non-volatile components in other parts of the plant (Borroto et al., 2008, 2010). This plant has some medicinal properties reported by several authors (Hernández & Volpato, 2004; Borroto et al., 2008, 2010).
Solid-phase microextraction (SPME) is a rapid, direct, inexpensive, solvent-free and efficient extraction technique for sampling different matrixes (Souza-Silva et al., 2015). In the field of food aroma analysis, the headspace SPME (HS-SPME) has proved to be an advanced and efficient tool for studies on food aromas, including fruits and fruit juices (Xu et al., 2016). In fact, HS-SPME combined with gas chromatography-mass spectrometry (GC-MS) is a convenient technique for providing the aroma profile of each analyzed fruit.
The aim of this study was to evaluate the changes of volatile compounds of the fruits of Morinda royoc L. at two ripening stages, using HS-SPME combined with GC-MS.
Materials and Methods
Sample and chemicals
Fruits of M. royoc were collected directly from plants grown in the coastal area of the city of Havana. They were selected at two maturity stages: ripe (peel with light amber color) and over-ripe (peel with dark brown color). Two groups, six fruits each one, were cut and seeds removed. The pulp was manually mashed analyzed immediately after sample preparation.
Authentic reference compounds were purchased from Sigma-Aldrich (Steinheim, Germany) and Merck (Darmstad, Germany), and some others were generously given by Robertet (Grasse, France). A normal paraffin solution (C5-C24) was supplied by Sigma-Aldrich (Steinheim, Germany). Sodium chloride was provided by Merck (Darmstadt, Germany).
Headspace-solid phase microextraction
A similar procedure reported earlier was used for the HS-SPME of the volatile compounds (Pino et al., 2010). A manual holder (Supelco, Inc., Bellefonte, PA, USA) and a 1-cm long, 65 μm polydimethylsiloxane-divinylbenzene (PDMS/DVB) (Supelco, Inc., Bellefonte, PA, USA) were used. For each extraction, 5 g of the homogenized pulp and 10 mL of 20 % NaCl solution (to inhibit enzymatic reactions and to favor the transfer of the analytes from the aqueous solution to the headspace) were centrifuged at 3000 min-1 for 10 min and the supernatant was transferred to a 15-mL Teflon-lined septum cap vial equipped with a Teflon-coated magnetic bar. The extraction was carried out under magnetic stirring at 600 min-1 at 40 °C for 20 min, after equilibration of the samples for 10 min at the same temperature. The fiber was then removed and introduced into the injector port of the GC for desorption at 250 °C for 2 min, in the splitless mode.
Gas chromatography-Mass spectrometry
GC-MS analysis was carried out using a Shimadzu QP-2010 Ultra (Kyoto, Japan), equipped with a 30 m x 0.25 mm i.d., 0.25 (m film thickness DB-Wax capillary column (J&W Scientific, Folsom, CA, USA). Thermal desorption was carried out at 250 oC in splitless mode for 2 min, using and inlet liner of 0.75 (m i.d. Carrier gas was helium at 1 mL/min. Oven temperature was held at 50 oC for 2 min, increased at 4 oC/min up to 250 oC and held for 3 min. The MS worked in electron impact mode at 70 eV ionization energy and in scanned mode from m/z 35 to 350, at 1.3 scan/s. Ion source and connecting parts temperature, 250 oC.
Identification of compounds was achieved by comparison of their linear retention index and mass spectra with those shown by reference standards when they were available. In other cases, comparison was made with those in commercial databases (NIST 05, NBS 75 k, Wiley 6 and Adams 2001).
Linear retention indexes were calculated using a mixture of normal paraffins. Semiquantitative determinations were based on the internal normalization method assuming similar calibration factors for all the compounds.
The original data from triplicate analyses were transformed as arc sen p ½, in which p is the proportion of each compound and processed for statistical analysis by the t-Student’ test.
Results and Discussion
The volatile constituents of M. royoc fruits were obtained by HS-SPME and analyzed by GC-MS using a polar fused silica capillary column. The peak areas of the compounds analyzed in triplicate on the same sample showed a coefficient of variation ≤ 5 %.
In total, 137 volatile compounds were identified, all of them for the first time, in M. royoc fruits (Table 1). These compounds comprise 42 esters, 22 acids, 17 alcohols, 14 ketones, 14 terpenes, 9 aldehydes, 9 sulfur-compounds, 4 lactones, 3 phenols and derivatives, 3 paraffins and a sulfur-nitrogen compound (benzothiazole).
Qualitative and quantitatively, acids were a major chemical class of compounds in both maturity stages, mainly in the ripe stage. They are represented by linear carboxylic acids generated from fatty acid biosynthesis, branched acids such as 2-methylpropanoic, 2-methylbutanoic, 3-methyl-3-butenoic, and 2-ethylhexanoic acid related to the synthesis of aliphatic amino acids, unsaturated aliphatic acids produced by the peroxidation of lipids and (E)-cinnamic acid as the only aromatic acid (Sanz & Pérez, 2010). Of them, octanoic acid and hexanoic acid were the major acids at both maturity stages. Both acids were also found as the most abundant in M. citrifolia fruits (Pino et al., 2010b). According to their odor notes (Burdock, 2010), both acids contribute to the noticeable rancid cheese odor of M. royoc fruit.
Esters were the second most abundant chemical class of compounds in both maturity stages, mainly in the over-ripe stage. Volatile esters are formed by esterification of alcohol and carboxylic acid through the enzymatic routes previously described (Sanz & Pérez, 2010). Alkenyl esters were the major ones in this class, particularly those related to 3-methyl-3-buten-1-ol and 3-methyl-2-buten-1-ol. Among these esters, 3-methyl-3-butenyl acetate, 3-methyl-3-butenyl butanoate, and 3-methyl-3-butenyl hexanoate were the major ones. These esters have powerful fruity odor notes (Burdock, 2010). In the previous study about the volatile composition of M. citrifolia fruits, 3-methyl-3-buten-1-yl hexanoate and 3-methyl-3-buten-1-yl octanoate were found the major esters (Pino et al., 2010b).
Some lactones, as intramolecular esters of 4-hydroxy acids, were also detected. They comprise (-butyrolactone, (-hexalactone, (-octalactone and (-decalactone. The biosynthesis of lactones is associated with the β- oxidation pathway and they are considered important contributors to the aroma of many fruits (Sanz & Pérez, 2010), but their contribution to the aroma of M. royoc fruits seem to be improbable due to the low contents.
Several aliphatic (13) and aromatic alcohols (4) were also detected. Like carbonyls, two main biogenetic pathways can be considered for alcohols: amino acid metabolism and lipid peroxidation pathways leading to unsaturated alcohols (Sanz & Pérez, 2010). The branched unsaturated alcohol, 3-methyl-3-buten-1-ol, was the most abundant in both maturity stages.
Amongst the identified carbonyl compounds, 9 aldehydes and 14 ketones were found. All of them are produced via lipid or aromatic amino acid metabolism (Sanz & Pérez, 2010). Of them, only heptan-2-one was present in notable amounts in the fruit at ripe stage.
Terpenes, although comprised 14 compounds, were present in small quantities at both maturity stages, excepting linalool in the ripe stage. Its contribution to the fruit’s aroma could be considerable due the low odor threshold of this monoterpene alcohol, which possess an intense floral odor (Burdock, 2010).
Interestingly, nine sulphur compounds were found in M. royoc fruit, including thiomethanol, dimethyl disulfide, dimethyl trisulfide, three S-methyl thioesters, 3-(methylthio)propanoic acid and two esters of this acid. In M. citrifolia fruit only dimethyl sulfide, dimethyl trisulfide and 3-(methylthio) propanol were found (Pino et al., 2010b). Benzothiazole was the only nitrogen-containing compounds in this fruit.
Three phenols and derivatives and three n-paraffins were identified, all of them at very low amounts.
Many significant differences were found based on the quantitative distributions of the compounds in both ripening stages. According to the chemical class, major changes noticed were the increase in esters contents and a decrease in acids, aldehydes, ketones and terpenes (Table 1).
Table 1 Volatile compounds (%) of Morinda royoc fruits at two ripening stages. (cont.)
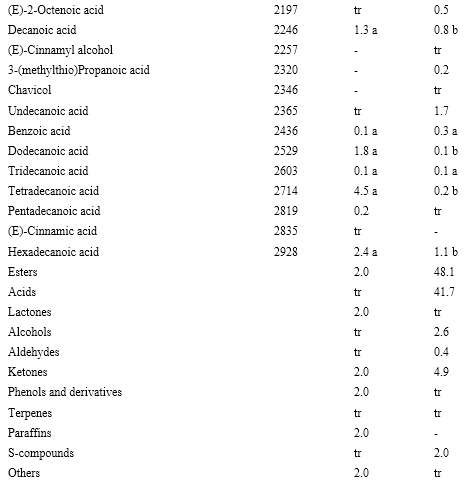
LRI: Lineal retention index in DB-Wax column. tr: <0.1%. Different letters in the same row indicate significant difference (p ( 0.05).
Although some compounds remain without changes in both ripening stages, the composition of certain volatile compounds clearly differs in both stages. Some esters, with fruity odor notes, increased, while some compounds, mainly acids, decreased or even disappeared during maturation. The over-ripe noni fruit showed significantly higher amounts of 3-methyl-3-butenyl butanoate, 3-methyl-3-butenyl hexanoate and 3-methyl-3-butenyl octanoate, while 3-methyl-3-butenyl acetate, benzyl acetate and benzyl butanoate significantly decreased. These changes probably indicate that esterification occurs during maturation, in a similar way as in other fruits (Sanz & Pérez, 2010). On the other hand, butanoic acid and 2-methylbutanoic acid increase their content, while the major part of the other acids decreased during maturation.
Conclusions
A total of 137 volatile constituents of Morinda royoc fruits at two ripening stages, all of them for the first time, were isolated by headspace solid-phase microextraction and analyzed by gas chromatography-mass spectrometry. Both ripening stages had several compounds in common. The compounds comprised 42 esters, 22 acids, 17 alcohols, 14 ketones, 14 terpenes, 9 aldehydes, 9 sulfur-compounds, 4 lactones, 3 phenols and derivatives, 3 paraffins and a nitrogen compound. Higher amounts of 3-methyl-3-butenyl butanoate, 3-methyl-3-butenyl hexanoate and 3-methyl-3-butenyl octanoate were present in the over-ripe fruits, while 3-methyl-3-butenyl acetate, benzyl acetate and benzyl butanoate significantly decreased. The content of butanoic acid and 2-methylbutanoic acid increased from mature to over-ripe stage, while the major part of the other acids decreased during maturation














