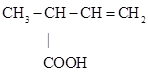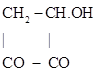Introduction
Life and career (Étard, 1904; Geneanet, 2019)
Eugène Anatole Demarçay (Figure 1) was born in Paris on January 1, 1852, the son of Camille Demarçay (1815-1893) and Cécile Lainé (1829-1916), and grandson of Marc-Jean Demarçay (1772-1839), a general during the French Revolution and a member of the French Parliament after the Renovation. He took his basic education at the Lycée Condorcet and after graduation he traveled in England for one year. Upon his return (1870) he enrolled in the École Polytechnique where he studied under Jean Baptiste Dumas (1800-1884) and worked as préparateur and répetiteur of Auguste Cahours (1813-1891). After graduation he traveled to Algeria, Egypt and India to study the geology and culture of foreign lands. Upon his return he joined the laboratory of Cahours and published his first scientific papers about the organic compounds of titanium and the composition and derivatives of chamomile (Demarçay, 1873a b, 1875a b, 1876a b c). In 1880 he was awarded his doctorate by the Faculté des Sciences of Paris, after successfully defending a
thesis about tetric and oxytetric acids and their homologues (Demarçay, 1880a). The results of his thesis were the main reason for his being awarded the 1880 Jecker Prize by the Académie des Sciences of Paris. As customary for candidates to the Académie, he published a booklet describing the results of his scientific research (Demarçay, 1883, 1904). His following researches were in organic and inorganic chemistry, particularly the synthesis and properties nitrogen sulfides. During his work on nitrogen sulfide he lost an eye as a consequence of an explosion. Afterwards he moved into spectroscopy and study of the rare earths and their spectrum. Among his many achievements in these subjects we can mention the design of a very sophisticated and accurate spectrometer, the discovery of the element europium, and the presence of radium in a sample provided by Marie Curie (1867-1934) and Pierre Curie (1859-1906).
Demarçay was married to Jeanne Berard (1864-1933); three children were born of this union: Genevieve (1890-1978), Marc (1893-1979), and Sabine (1897-1991).
Demarçay passed away in Paris on March 5, 1903, after long suffering the effects of radium radiation.
Scientific Contribution
Demarçay wrote about 50 papers on the subjects of inorganic and organic chemistry, natural products, rare earth and radioactive elements, and spectroscopy. In addition to the subjects described below he also prepared derivatives of acetylvaleric acid, mono- bi- and trichloroacids, acetoacetic esters, monochlorinated acids of the acrylic series, and isobutylacetocetic acid (Demarçay, 1876b, 1877a b c, 1878a b); he identified the hydrocarbons and low weight fatty acids generated during the steam distillation of the crude fatty acids originating from the saponification of fats (Cahours & Demarçay, 1875, 1879c, 1880); studied the reaction between oxalic acid and alcohols (Cahours & Demarçay, 1876, 1878); the formation of tin organometallic derivatives with alcohols (Cahours & Demarçay, 1879a b), the preparation of acetonitrile (Demarçay, 1880d); the possible values of the valence of sulfur (Demarçay, 1881b); he synthesized the various hydrates of thorium sulfate (Demarçay, 1883a) and many derivatives of tellurium (Demarçay, 1883b); developed an efficient method for separating titanium from niobium and zirconium (Demarçay, 1885a), and a very precise method for detecting the presence of rhodium (Demarçay, 1885b); demonstrated the presence of vanadium, molybdenum, and chrome in vegetables (Demarçay, 1900a); etc.
Titanium Organic Derivatives
According to Demarçay, titanium presented several analogies with silicon and tin; for example it formed an oxide TiO2 similar to silica SiO2 and stannic anhydride, SnO2, and its volatile chloride, TiCl4, was similar to the corresponding chlorides SiCl4 and SnCl4. Tin chloride was known to form many combinations with esters, hence it seemed of interest to investigate the parallel behavior of titanium. In his first paper on the subject, Demarçay wrote that titanium chloride was able of combining with the oxygenated ethers (esters) and with the alcoholic sulfides and hydrosulfites (Demarçay, 1875a). The action of this chloride upon ethyl acetate, butyrate, valerate, caproate, angelate, benzoate, oxalate, succinate, amyl acetate and valerate, and methyl benzoate, had already been reported. The combinations of titanium chloride with the esters of monoacids could be identified by the three formulas (TiCl4)2E, TiCl4E, and TiCl4EE', where E and E' represented the same or a different equivalent, and those of dibasic acids, by (TiCl4)4E, (TiCl4)2E, and TiCl4E. Demarçay described several synthesis procedures for obtaining the pertinent derivatives. Those of the first group could be prepared by adding drop-wise the calculated amount of ester to titanium chloride; those of the second group were prepared by adding to the purified products of the first group a calculated amount of ester. All these reactions were highly exothermal and the resulting products were slightly yellow non-volatile solids. They could only be obtained by fractionated fusion over an oil or water bath. They were heat labile, leaving a carbonaceous residue. Upon distilled, they passed ill-defined liquid. Water, alcohol, and humidity decomposed them into the corresponding ester (Demarçay, 1873a).
Demarçay added the these compounds were hardly obtained as isolated crystals, although they usually appeared as crystalline masses not presenting net crystalline shapes. Combinations of the type TiCl4E did not solidify but could be recovered as such from their efflorescence. Demarçay believed that these compounds could be considered as chlorhydrins analogous to the silicic chlorhydrins of Charles Friedel (1832-1899) and James Mason Crafts (1839-1917) (Friedel & Craft, 1870), united to the chlorides of acid radicals. In other words, they constituted a sort of double chlorides. According to this hypothesis, titanium chloride, for example, generated the following acetates (attention must be paid to the fact that Demarçay was using the old values of atomic masses: C = 6, oxygen = 8 and hydrogen monoatomic):
(1) (Ti2Cl4)2C4H3O4C4H5
(2) Ti2Cl4C4H3O4C4H5
(3) Ti2Cl4(C4H3O4C4H5)2
Demarçay explained that the reason behind this viewpoint was that the known trichlorhydrin, Ti2O2C4H5.Cl3, combined directly with the chlorides of acid radicals and with titanium chloride, to yield compounds of the first group (Demarçay, 1873a).
Demarçay also provided the equations of the complete series of derivatives (5 in number) of benzoate. He also stated that the sulfides and thionates of alcoholic radicals behaved with titanium chloride in the same manner as a normal ether:
Ti2Cl4,C4H5.HS2 = Ti2S2C4H5.Cl3,HCl
Ti2Cl4,(C4H5)2S2 = Ti2S2C4H5.Cl3,C4H5Cl
Ti2Cl4,(C4H5.HS2)2 = Ti2(S2C4H5)2Cl2,(HCl)2
Ti2Cl4,(C8H10S2)2 = Ti2(S2C4H5)2Cl2,(C4H5Cl)2
The first two compounds were red black, the third scarlet red, and the fourth dark red. Water, alcohol, and ethers decomposed all of them, with liberation of alkyl sulfide (Demarçay, 1873b).
In a second paper Demarçay mentioned that the only ethoxide known was titanium trichlorhydrin, TiCl3,OC2H5 (Demarçay, 1875a). It was prepared by reacting an equimolar mixture of alcohol or ether and titanium chloride. A similar process produced dichlorhydrin. These derivatives could be rapidly prepared as beautiful crystals by slowly adding one mole of titanium chloride to four moles of absolute alcohol and heating the mixture under vacuum from 800 to 100 0C. The excess alcohol and the released HCl were eliminated by distillation. The white crystalline residue was dissolved in a small amount of boiling alcohol; on cooling it deposited large brilliant crystals of monochlorhydrin hydrochloride, melting between 1050 and 110 0C and yielding a viscous liquid, which released HCl under vacuum and sublimed into crystals of unknown composition, although probably of the monochlorhydrin (Demarçay, 1875a).
Demarçay reported that a mixture of sodium ethoxide and enough alcohol reacted with trichlorhydrin hydrochloride to produce a precipitate of sodium chloride and crystals of a compound, which was found to be titanium pentaethylate, Ti(OC2H5)5. This compound was highly unstable, particularly in the presence of humidity or a very small amount of alcohol. In contact with air, its ethereal solutions became instantly turbid due to the precipitation of a little of titanic acid (Demarçay, 1875a).
Essence of chamomile
In 1848 Charles Gerhardt (1816-1856) reported that the essence of Roman chamomile (Anthemis nobilis) contained angelic acid (2-methylisocrotonic acid, z-isovaleric acid), valeric acid, a hydrocarbon of formula C10H16, which he named camomillene, and an oxygenated principle, which under the action of KOH originated the two acids. Camomillene was a citron smelling liquid, boiling at 175 0C, isomeric with turpentine, which did not react with fuming sulfuric acid. Heating the essence with KOH released hydrogen and produced angelic acid (Gerhardt, 1848).
In a paper published in1873 Demarçay mentioned that during the preparation of angelic acid from the essence of chamomile he had separated the liquid named camomillene and found that its properties were quite different from those reported by Gerhardt. This finding led him to make a more detailed study of the essence (Demarçay, 1873b).
The results of his experiments indicated that reaction of the essence of chamomile with KOH did not release the hydrocarbon noticed by Gerhardt. It was possible to decompose the essence without gas generation and always obtain the same products. The action of alcoholic KOH and fused KOH upon the oil produced identical results, provided the alkali was shaken with the oil several times, or until it had no further action. This latter process, however, occasioned considerable loss of the volatile products, leading Demarçay to prefer the former. Demarçay's process consisted in adding the KOH in small pieces to a solution of equal volumes of oil and alcohol, shaking the mixture until all the alkali had dissolved, and then leaving the whole mass for 36 hours. The resulting mass was mixed with water and then distilled until only waster passed over. Addition of potassium carbonate to the distillate split it into two liquid phases. The upper phase was first dried with potassium carbonate, then with anhydrous baryta, and afterwards distilled. The first liquid passed at above 100 0C and was found to be alcohol; the remaining material passed between 1050 and 160 0C. Further distillation of the second liquid separated it into two fractions, one boiling between 1070 and 109 0C, and the other between 1290 and 132 0C. These two phases were identified as butanol and amyl alcohol, respectively. Demarçay found that a small amount of distillate, passing at above 135 0C seemed to be a mixture of alcohols; a finding not reported by Gerhardt because he dried the liquid with calcium chloride, which combined with the alcohols (Demarçay, 1873b).
The results of additional tests led Demarçay to conclude that Roman chamomile was a mixture of several esters, mainly the valerates and angelates of butyl and amyl, and not of aldehydes (Demarçay, 1873b).
A following paper dealt with angelic acid dibromide and the possible structure of the acid (Demarçay, 1875b). It was known that angelic acid reacted with bromine to yield a dibromide; this compound reacted with KOH producing monobromobutylene and CO2. Demarçay found that the distillation of the dibromide resulted in the formation of a large amount of gas and oil that sometimes crystallized. Boiling a solution of the oil in KOH followed by addition of an excess of sulfuric acid produced oil that soon solidified into a crystalline mass. The purified colorless crystals melted at 610- 62 0C, boiled at 1940- 196 0C, and were slightly soluble in cold water and much more in boiling water, from were they precipitated as small lustrous needles. Elemental analysis indicated a composition equivalent to the formula C5H8O2, indicating that it was isomeric with angelic acid. These results showed that the new acid closely resembled the methylcrotonic acid synthesized by Edward Frankland (1825-1899) and Balwin Francis Duppa (1828-1873) (Frankland & Duppa, 1865). Demarçay also found a complete identity between brominated methylcrotonic acid and angelic acid dibromide; the first one boiled at 790 to 82 0C and the second at 770 to 80 0C (Demarçay, 1875b). He also supported Frankland's assumption that angelic acid was represented by a methylcyclopropyl acetic acid, although in a following publication he indicated that he believed that the correct formula was:
(double bond in the wrong position) (Demarçay, 1876c).
Oxypyrotartaric Acid
Demarçay wrote that acetoacetic acid was known to be a compound having a ketone and ester group: CH3-CO-CH2-COOC2H5, and for this reason he had used it to fix HCN on the ketone group (Demarçay, 1876a). For this purpose he heated on a water bath the ester with half its weight of anhydrous HCN for three hours. The resulting mixture was cleaned of the excess acid and afterwards heated with HCl, which resulted in the formation of a large amount of ammonium chloride. The excess HCl was eliminated by heating on a water bath, followed by extraction with ether and evaporation of the ether. The resulting thick syrup was oxypyrotartaric acid, as shown by the following reactions:
The resulting oxypyrotartaric acid was yet to be obtained in the crystalline form. Neutralization of the solution with baryta water, followed by boiling and treatment with HCl, resulted in the formation of a soluble acid, soluble in ether, that crystallized by evaporation under vacuum as colorless brilliant prisms, melting at 78 0C, sublimating easily, and forming a salt having all the properties and composition of zinc acetonate (zinc 2-hydroxyisobutyrate).
Demarçay added that oxypyrotartaric acid decomposed by distillation at 220 0C in a remarkable manner, yielding CO, CO2, water, citraconic anhydride, and a body having the properties of isopropyl alcohol. The reaction with nitric acid yielded an acid having all the properties of mesaconic acid (trans citraconic acid) (Demarçay, 1876a).
Dicarboxylic acids
In 1879 Demarçay published the first of a series of papers on dicarboxylic acids of general formula 3CnH2n-4O2 + H2O, related to the normal dicarboxylic acids succinic, glutaric, adipic, and pimelic acids (Demarçay, 1879a b c, 1880a). In this first paper he referred to the following members of the heptanedioic series:
(a) Tetric acid, 3C4H4O2 + H2O, a colorless body that precipitated as triclinic crystals upon evaporation of its aqueous solution, It was sparingly soluble in cold water, extremely soluble in alcohol, ether, and boiling water, insoluble in cold chloroform, and very soluble in boiling chloroform mixed with a few drops of alcohol. It melted at 189 0C without alteration and colored violet red a solution of ferric chloride (the same as its homologues).
(b) Pentic acid, 3C5H6O2 + H2O, appeared at orthorhombic crystals, melting at 1270-128 0C, little soluble in cold chloroform and very soluble in hot chloroform.
(c) Hexic acid, 3C6H8O2 + H2O, prepared from acetopropylacetic acid, colorless, melting at 126 0C, and precipitating as large silky plates from its boiling aqueous solution.
(d) Isohexic acid, 3C6H8O2 + H2O, obtained from ethyl acetoisopropylacetate and precipitating as voluminous orthorhombic prisms melting at 124 0C.
(e) Heptic acid, 3C7H10O2 + H2O, little soluble in cold water, very soluble in boiling water, in alcohol, ether, and chloroform, melting at 1210-123 0C, and boiling at about 260 0C (Demarçay, 1879a).
All these acids had very similar properties: They decomposed above 300 0C while blackening and passing a non-decomposed fraction. They formed salts having very similar composition; the radicals CnH2n-4O2 behaved like silicic anhydride and many of them combined with one or more molecules of a base. Fuming nitric acid attacked them forming crystallizable nitrate derivatives, they dissolved in sulfuric acid without change, and added bromine and chlorine yielding liquid compounds that were not decomposed by cold water (Demarçay, 1879a).
In the following paper Demarçay described the homologues of oxyheptic acid he had prepared (Demarçay, 1879b):
(a) Oxytetric acid, 3C4H4O2 + H2O: crystallizing as small spherical mamelons formed of small needles, deposited by cooling from its solution in boiling water, melting at 2030-204 0C, very soluble in alcohol and ether and insoluble in chloroform.
(b) Oxypentic acid, 3C5H6O2 + H2O: melting at 193 0C and crystallizing the same as oxytetric acid.
(c) Oxyhexic acid, 3C6H8O2 + H2O: derived from ethyl propylacetoacetate, melting at 1730-174 0C and precipitating as small silky plates from a boiling solution.
(d) Isohexic acid, 3C6H8O2 + H2O: isomer of oxyhexic acid and derived from ethyl isopropylacetoacetate, melting at 1860-187 0C and precipitating as clinorhombic crystals by slow evaporation of an aqueous solution (Demarçay, 1879b).
All the above compounds behaved as strong acids; they easily decomposed the carbonates, esterified easily with alcohol at 150 0C, and were easily hydrogenated according to the following equation:
(3C4H4O2 + H2O) + H2 = 3C4H6O2 + H2O
yielding, in this example, hydroxytetric acid, melting at 111 0C, crystallizing easily, very soluble in water, alcohol, and ether. These acids were also esterified by alcohol at 150 0C yielding esters with an agreeable slight odor (Demarçay, 1879b).
According to Demarçay, all the above information pointed clearly the structure of the new acids (Demarçay, 1879c). For example; the nature of the radical C4H4O5 of oxytetric acid had to be the same as that of the radical of malic acid
The presence of the group OH was proved by the fact that oxytetric acid reacted with phosphorus pentachloride to yield the chloride C4H3Cl3O. Hence, the structure of the higher homologues (pentic, oxypentic, etc.) had to be the same as the radical of succinic acid where one or two methyl, ethyl etc. groups had replaced one or two hydrogen atoms (Demarçay, 1879c).
The last paper was a long memoir (50 pages) summarizing Demarçay's doctoral thesis on the subject (Demarçay, 1880d). The first part gave a detailed description of all the chemical reactions leading to the different new derivatives, starting from ethyl acetoacetate. The following section gave a general description of the synthesis of tetric acid and its homologues and the precautions to be taken. The third and fourth sections described the different byproducts of the main reactions, their chemical formula, and properties, and a general description of the preparation of oxytetric acid and its homologues. The bulk of the remaining part of the report was a detailed description of the preparation and properties of the different acids, their salts, and derivatives. The last pages described the experiments performed to prove the suggested structure of the radicals. Demarçay wrote that all were based on the assumption that carbon was tetratomic, oxygen diatomic, and hydrogen monatomic. All the reactions were assumed to be simple double exchanges between the atomicity of the reacting reagents without any transposition of the diverse elements of a given combination while it reacted with another (Demarçay, 1880a).
Nitrogen sulfide
In 1837 Eugène Soubeiran (1797-1859) reported that he had prepared nitrogen sulfide by reacting gaseous ammonia with sulfur chloride. This new compound was a lemon yellow odorless solid, which detonated violently by percussion or by the sudden application of heat, and contained three atoms of sulfur and one of nitrogen, NS3 (Soubeiran, 1837, 1838). August Laurent (1807-1853) published a paper claiming, among other things, that the compound nitrogen sulfide did not exist and that the substance, which had been improperly named so, contained hydrogen and had for composition S2N2H2 (Laurent, 1849). Mathurin-Joseph Fordos (1818-1878) and Amédée Gélis (1815-1882) criticized Soubeiran's formula because it meant that three equivalents of water combined with one equivalent of nitrogen sulfide to yield an ammonium sesquihyposulfite of formula S3O3,NH4,Aq, a result that contradicted their findings that all known neutral hyposulfites had the general formula S2O2,MO (Fordos & Gélis, 1850, 1851). Fordos and Gélis prepared nitrogen sulfide by bubbling ammonia gas through a solution of sulfur chloride in carbon disulfide. The compound crystallized in transparent golden-yellow rhombic prisms, which should be pulverized carefully in very small quantities because the least percussion caused it to explode violently. Elemental analysis indicated that it contained, by weight, 69.34% of sulfur and 30.66% of nitrogen, corresponding very closely to the formula NS2. Hence it did not contain hydrogen and was not similar to ammonia. They also studied the reaction of nitrogen sulfide with water, the alkalis and sulfur chlorides, and reported the preparation and properties of derivatives such as SCl,NS2, SCl,2(NS2), and SCl,3(NS2), which they named nitrogen sulfide chlorosulfates (Fordos & Gélis, 1850, 1851).
Demarçay studied the action of chlorine on nitrogen sulfide (Demarçay, 1880b). He noticed that when nitrogen chloride (wetted with chloroform to avoid possible increases in temperature resulting from direct reactions) was treated with a stream of chlorine it dissolved slowly, with release of heat. The color of liquid changed from originally red orange to green olive, and then to black when the temperature was substantially higher, and to red brown when the reaction was over. The liquid, after cooling, deposited beautiful crystals of NSCl, which on heating decomposed into nitrogen and sulfur monochloride. The dark coloration observed during the preparation of this chloride was due to the formation of a second chloride. This second chloride was better prepared by dissolving NSCl in chloroform and adding double the corresponding amount of nitrogen chloride it contained. This chloride, of formula (NS)3Cl, crystallized as long copper red needles. According to Demarçay, his results indicated that the combinations discovered by Fordos and Gélis could be written as follows (Demarçay, 1880b):
2(SCl2,NS) = (SNCl)2.S2Cl2
2(SCl2,3NS) = [(SN)3Cl]2.S2Cl2
2(SCl2,2NS) = [SNCl,(SN)3Cl].S2Cl2
Additional compounds could be prepared by using S2Cl2 in great excess, dissolved in chloroform. The resulting liquid deposited a yellow crystalline powder, insoluble in most solvents, except chloroform and boiling thionyl chloride, SOCl2. On cooling the later, it deposited the compound S4N3Cl, as a result of the reaction
3N2S2 + S2Cl2 = 2S4N3Cl
This compound was more stable to heat than nitrogen sulfide; it did not decompose in contact with dry air and did it very slowly in humid air. It dissolved in concentrated nitric acid without apparently reacting. Evaporation of the solution under vacuum left a residue of citron yellow crystals of formula S4N2NO3. The compound S4N3Cl reacted with concentrated sulfuric acid, releasing HCl and leaving a yellow solution that did not decompose with time. Treatment of the solution with acetic acid deposited a new compound of formula S4N2HSO4.
According to Demarçay, his results indicated that the radical S4N2 was able to function as a base or like an alcoholic radical, which could be represented by the formula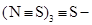 , similar to the sulfines of August Cahours (1813-1891), for example, trimetylsulfine,
, similar to the sulfines of August Cahours (1813-1891), for example, trimetylsulfine, 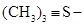 (Cahours, 1865). Demarçay named the three new compounds he had synthesized thiotrithiazyl chloride, nitrate, and bisulfate, respectively, and thiazyl the radical SN (Demarçay, 1880c).
(Cahours, 1865). Demarçay named the three new compounds he had synthesized thiotrithiazyl chloride, nitrate, and bisulfate, respectively, and thiazyl the radical SN (Demarçay, 1880c).
According to Demarçay the reaction between sulfur monochloride and nitrogen sulfide produced different products according to the operating conditions (Demarçay, 1881a). Treating sulfur chloride with hot nitrogen sulfide resulted in the formation of the chloride of the radical S4N2Cl. Under cold, the reaction between sulfur chloride and nitrogen sulfide dissolved in chloroform generated voluminous crystals but, if it took place between cold sulfur chloride and solid nitrogen sulfide, the product was a black crystalline powder of dithiotetrathiazol dichloride, according to the equation
2S2N2 + S2Cl2 = S6N4Cl2
This compound was highly unstable, slight heating or addition of nitric acid, decomposed it violently (Demarçay, 1881b).
Addition of thiazyl chloride, SNCl, to sulfur monochloride resulted in a limpid liquid that after sometime became brown and deposited yellow voluminous crystals, corresponding to the formula S3N2Cl2, thiodithiazyl dichoride, formed according to the equation
4SNCl + S2Cl2 = Cl2 + 2S3N2Cl2
Fordos and Gélis obtained this compound by reacting nitrogen sulfide with sulfur dichloride. Its reaction with water produced the black substance S2N2O2 (Fordos & Gélis, 1850).
All the work of Demarçay with nitrogen sulfide had a tragic end; an explosion of one the derivatives caused him to lose one eye.
Volatilization of metals
It was known that metals could be volatilized a very high temperatures and at atmospheric pressure, usually by means of the electric arc. Demarçay decided to study the possibility of carrying on the process at low pressure, particularly under vacuum. His results justified this possibility (Demarçay, 1882). Basically, his equipment consisted of a crystal tube of diameter 20 mm, closed at one end, containing the metal being examined, heated by means of vapors of different substances (i.e. sulfur, mercury, aniline, methyl oxalate, etc.) capable of achieving temperatures between 100 0 and 440 0C, and connected to a vacuum pump. The metals tested were cadmium, zinc, antimony, bismuth, lead, and tin. The results indicated that the metals were volatile at temperatures well below their melting points: cadmium 160 0C, zinc 184 0C, antimony and bismuth 292 0C, and lead and tin 360 0C. The metal deposits achieved were quite considerable, particularly at high temperatures. For example, cadmium at 184 0C deposited 0.1 g after 24 hours of operation (Demarçay, 1882).
Action of carbon tetrachloride on oxides
In 1865 Paul Schützenberger (1829-1897) reported that SO3 reacted with an excess of carbon tetrachloride to produce gas phosgene and a colorless liquid (pyrosulfuryl chloride), fuming on air, boiling at 130 0C, and decomposed by water into HCl and sulfuric acid. Elemental analysis indicated that its composition corresponded to the formula S2O5Cl2. The reaction could be represented as follows:
This equation could be generalized so that with chloroform it would be
that is, yielding pyrosulfuryl chloride and formyl chloride (Schützenberger, 1869).
Demarçay decided to study the possibility that carbon tetrachloride reacted with oxides according to one of the two following equations:
so that the reaction could be used in laboratories to produce anhydrous chlorides (Demarçay, 1887a). The experimental results confirmed this assumption: carbon tetrachloride reacted easily with the oxides of chrome, aluminum, titanium, niobium, tantalum, and zirconium, but not with silica. The only limitation was that the reaction had to be carried below the temperature of decomposition of carbon tetrachloride, that is, a temperature below red (about 550 0C). For example, the reaction with niobium pentoxide occurred slowly at the boiling point of naphthalene (220 0C) and very rapidly at 440 0C. Titanium dioxide also reacted easily at 440 0C (Demarçay, 1887a)
Spectrum techniques
Demarçay wrote that the spectroscopic method of Paul-Émile Lecoq de Boisbaudran (1838-1912) was widely employed, in spite of its limitations (Demarçay, 1884). It employed the light produced by the high potential spark of a wire induction armature coil having a long and thin wire. This was necessary because the liquid opposed the spark with a resistance that a normal spark was unable to overcome. Unfortunately, with certain substances the light of the resulting spark did not produce an adequate spectrum. This limitation had forced some researchers to try to determine the lines of these substances using an induction coil connected with a rather powerful capacitor battery. This arrangement was particularly important when it was desired to generate the spectrum of metallic electrodes; otherwise the light of the resulting spark was insignificant. Regrettably this procedure did no avoid the presence of secondary lines originating from the air, and from the lines of the electrodes. In particular, the brightness of the spectrum of air was substantially stronger than that of the metallic line and reduced the value of the desired spectrum (Demarçay, 1884).
Demarçay built an apparatus that eliminated these limitations by employing the spark produced by an induction coil provided with a short and thick platinum wire. This arrangement produced a spark of only 5 mm long, under the action of a pile containing 6 to 9 elements of potassium dichromate. The coil was 23 cm long and 11.5 mm internal diameter; the inducting and induced wires had 4 and 1 mm diameter, respectively. According to Demarçay, the best results were obtained using a concentrated solution of the substance of interest in a solvent as stable as possible. The apparatus allowed obtaining without difficulty the spectra of all simple substances. In general, the spectrum contained fine lines where the violet portion was extremely brilliant. The spectrum of sulfuric, selenic, phosphoric, and arsenic acids yielded the lines of sulfur, selenium, phosphorus, and arsenic, although the presence of minimum amounts of the metal in solution was enough to make the lines disappear. The spectrum of hydrogen was represented by two red and blue lines, very neat and without interference (Demarçay, 1884).
Spectrum of didyme and samarium
In 1886 Demarçay announced that an examination of the absorption spectra of several of the products of the fractionation of didyme and samarium had led him to new significant results (Demarçay, 1886). It was known that the violet and ultraviolet zones of the spectrum of samarium contained the bands described by Lecoq de Boisbaudran (Lecoq de Boisbaudran, 1879) and Jacques Louis (1827-1890) (Soret, 1880), having wavelengths 419-415, 407, 400, and 374. Demarçay observed that the bands 417 and 374 could be neatly separated of the bands 407 and 400. This indicated that the samarium defined by these four bands contained actually at least two simple bodies. He kept the bands 407 and 400 for the element defined as samarium and named the other element, temporarily, by the symbol S. He also wrote that didymium (dysprosium) had been separated by Carl Auer von Welsbach (1858-1929) from praseodymium, neodymium, and a third element about which he had said nothing but admitted its existence by eliminating from the spectra of neodymium and praseodymium one of the lines (( about 476), which seemed not to belong to none of these two elements (Auer von Welsbach, 1886). Demarçay studied the spectrum of many fractions and found another very narrow line (( about 434), not pointed out, which together with ( = 476, he believed belonged to the third element (Demarçay, 1886).
In a following short note, Demarçay confirmed his result that in the spectrum of samarium the band ( = 417 did not belong to the substance that provided the blue bands ( = 480 and 463. The products of the fractionation contained earths very rich in praseodymium as well as earths that did not encompass the element. The fractionated earths confined between pure praseodymium and neodymium contained, in equal intensity, the bands ( = 444 and 469, attributed by Auer von Welsbach to praseodymium, with an intensity clearly higher to those present in the earths of praseodymium almost pure. The necessary conclusion was that 469 belonged to a different body (Demarçay, 1887b). In a following note Demarçay added that in the portions of neodymium free of praseodymium and containing a small amount of samarium he had found the following lines that had yet to be identified: (a) ( = 464, very thin, on the little refrangible edge of the nebulous line ( = 461.8 of dysprosium; (b) ( = 430, a weak and thin line, and (c) a line at approximately ( = 464 of the spectrum of dysprosium in nitric solution, composed of a strong double band where the most refrangible component was also the most intense, the corresponding wavelengths having ( = 473.4 and ( = 476 (Demarçay, 1887c).
In 1878 Jean Charles Galissard de Marignac announced the discovery of a new earth contained in the rare earths near samarium, and which he named Y( (afterwards, gadolinium) (Marignac, 1878). Demarçay investigated this matter further and reported that by fractional crystallization in fuming nitric acid of density 1.45, he had separated a colorless nitrate, little soluble in cold water, showing very weak trances of the absorption bands of samarium, and with the spark, a rich spectrum of gadolinium (Demarçay, 1896). Further crystallizations produced fractions colored yellow orange intense. This material showed no traces of the lines and bands of gadolinium. According to Demarçay, this material differed from the known rare earths in producing colorless salts without absorption spectrum, in being different from terbium, and in having a spectrum different from the oxides of lanthanum, cerium, gadolinium, ytterbium, and terbium (the only known rare earths having colorless salts). It was also quite different from the oxides of lanthanum and cerium in generating a weakly basic sulfate and a relatively soluble double sulfate with potassium. Demarçay named ( the radical of this new earth, and (2O3 the earth itself. He also believed that another new earth was also present, having characteristic lines at 4228.1, 4205.9, 4128.4, 3972.2, 3930.8, and 3819.9 (Demarçay, 1896).
In a following paper, Demarçay announced that using a fractionated procedure based on the manufacture of double magnesium-rare earth nitrates he had been able to concentrate his ( element to such levels that now its spectrum contained lines as bright as those produced by barium and yttrium (Demarçay, 1900b). The spectrum presented three particular blue lines, which seemed to belong to another non-identified rare earth described by Lecoq Boisbaudran under the symbol Zg. Comparison of the lines of both earths, ( and Zg, suggested that they were identical; hence Demarçay suggested naming it (-Zg (Demarçay, 1900b). Demarçay reported that the oxides and salts containing the highest concentration of the new element had a pale rose color and exhibited the characteristic absorption, spark, and reversal spectra. Its atomic mass, obtained from the synthesis of the sulfate, was approximately 151. This paper contained a detailed list of the spectrum lines of (-Zg (Demarçay, 1900b). Demarçay wrote that in 1885 William Crookes (1832-1919) detected a band that he attributed to samarium; this band presented the anomalous property of disappearing in the presence of calcium sulfate. In 1889 he distinguished this line and many other bands as characterizing a special meta element that he called S3 (Crookes, 1886, 1889). In 1892 Lecoq de Boisbaudran described a spectrum of three brilliant blue lines present in the spark spectrum of samarium. He concluded that they corresponded to a particular element that he named Z3. Sometime later he found a particular band in the reversal spectrum of samarium, which he believed belonged to a particular element that he named Z( (Lecoq de Boisbaudran, 1892, 1893). Demarçay added that in 1896 he had also announced the existence of an element intermediate between gadolinium and samarium, characterized by strong violet and ultraviolet lines and in 1900 he had showed that this element was identical with the Zg of Lecoq de Boisbaudran and probably the same as that announced by Crookes (Demarçay, 1896, 1900b). Demarçay was able to prepare large quantities of the new element, very pure, using the fractionating method based on the double magnesium-rare earth nitrates procedure (Demarçay, 1901). Its spectrum contained three main bands: ( = 609, very strong; ( = 593, strong and large; and ( = 576, notably and large. He also noticed that addition of traces of calcium sulfate gave a brilliant fluorescent spectrum that dominated the anomalous line of Crookes. According to Demarçay, the apparently contradictory results of Crookes and Lecoq de Boisbaudran were due to the extremely small quantity of (-Zg present in their sample and the fact that calcium and gadolinium reinforced more the spectrum of samarium than that of gadolinium. Demarçay proposed naming the new element, europium. This paper contained a detailed list of the electric lines (21) of europium comprised between ( = 5000 and ( = 3500 (Demarçay, 1901).
Demarçay summarized in a book all the spectra information about the rare earth elements (Demarçay, 1895).
Radioactive elements
In 1898 Marie Curie (1867-1934) and Pierre Curie (1859-1906), asked Demarçay to study the spectrum of a substance containing barium chloride, which they believed contained a new element (Demarçay, 1898). This substance, dissolved in distilled water slightly acidulated with HCl, and submitted to the action of the coil developed by Demarçay, produced a brilliant spectrum, which was photographed. Demarçay measured the lines and observed the following facts: (1) barium was represented by the large intensity of its strong and weak lines; (2) lead was identified by it principal lines, weaker than the others; (3) the lines of the platinum of the two electrodes and the main lines due to the solvent; and (4) an outstanding line, stronger than the weak lines of barium at ( = 3814.8, which could not be attributed to any known element. On the basis of these findings Demarçay concluded that the presence of this particular line justified the presence of a new element (radium) in the barium chloride provided by the Curie's (Demarçay, 1898).
Sometime afterwards, Marie Curie provided Demarçay with a sample of radium chloride she had prepared, for further inspection of its spectrum. Demarçay found that the spectrum of a diluted HCl solution of this salt presented the lines of (a) the platinum electrodes; (b) a weak spectrum of barium reduced to its principal roots at ( = 4554.4, 4130.8, and 3892.2. No other lines could be attributed for radium above the ones listed in the previous publication (Demarçay, 1900c)