INTRODUCTION
Phenanthrene (FNN) is a recalcitrant polyaromatic hydrocarbon (PAH) usually present in sites impacted by crude oil, but also several FNN derivatives have been reported as plant natural products with biological activities (Tóth et al., 2017). Quercetin (QTN) is a natural flavonoid, that owing to its high antioxidant value (ORAC µmolTrolox/mg = 21.45 ± 1.17), as reported by Miled et al. (2017), and Zhang et al. (2013), and its low ionization energy (0.10V), reported by Li et al. (2020), may be able to activate the oxidation process of recalcitrant compounds, such as PAH, acting as chemical mediator in the dynamic of removal of PAH by phytoremediation processes in sites impacted with such compounds (Calva et al., 2016). In the enzyme-mediator-substrate (EMS) systems, a chemical mediators (M) is a small electron-carrying molecule that after being oxidized by either an enzyme (E), such as laccases and peroxidases, or by reactive chemical species, such as alkyl, alkoxy, and peroxy radicals, or carbonyl oxides (Barber and Kroll, 2021), can be released to subsequently oxidize, through non-enzymatic reactions, other susceptible chemical substrate (S), which due to their chemical structure, molecular size or location, cannot be attacked by enzymes (Johannes and Majcherczyk, 2000; Baiocco et al., 2003; Camareno et al., 2008; Longoria et al., 2008; Torres-Duarte et al., 2009). There are several studies reporting that plant phenolic compounds can act as chemical mediators for the oxidation and removal of recalcitrant xenobiotics by EMS systems (Johannes and Majcherczyk, 2000; Baiocco et al., 2003; Camarero et al., 2008; Longoria et al., 2008; Torres-Duarte et al., 2009). For example, phenylpropanoids derived from the degradation of lignin have been used as chemical mediators for the oxidation and removal of xenobiotics by EMS systems (Baiocco et al., 2003). Similar mechanism using both synthetic and natural chemical mediators in combination with fungal enzymes for the removal of xenobiotics have been reported for the removal of polyaromatic hydrocarbons (Majcherczyk et al., 1998; Torres-Duarte et al., 2009). Similarly, previous results from our research group have suggested that QTN, and other flavonoids excreted by Cyperus laxus plants, might act as natural metabolic mediator in the dynamic of the phytoremediation processes for removal of PAH by using several EMS system (Rivera-Casado et al., 2010; Rivera-Casado et al., 2015b; Calva et al., 2016). From that research, plants cultivated for phytoremediation of recalcitrant oil impacted sites, the hydrocarbons removal was closely associated with changes in the fatty acid composition and the production of unknown conjugated compounds between some PAH and several plant phenolics, with predominance of phenylpropanoids and flavonoids (Rivera-Casado et al., 2010, 2015a, 2015b). So, it was hypothesized that plant metabolites with high antioxidant power, such as flavonoids, should have the ability to participate in the dynamic for the removal processes of PAH, acting as metabolic mediators in the mechanism of phytoremediation processes. Exploring such hypothesis, it was found that QTN is the predominant flavonoid in the phenolic profile of both greenhouse and in vitro plants of Cyperus laxus when cultivated in the presence of PAH (Rivera-Casado et al., 2010, 2015a), and that the content of this flavonoid was increased by FNN in in vitro cultures (Rivera-Casado et al., 2015a, 2015b, Calva et al., 2016). In continuation with this research, in this work, the removal of FNN and QTN by the EMS system, constituted by FNN (S), QTN (M), and radish peroxidase (E), in comparison with several control reaction mixtures omitting one or several components of the EMS system, and H2O2, was studied. The objective was to examine whether QTN, in the presence of FNN as PAH model, can act as metabolic mediator in the transformation of FNN by radish peroxidase as model oxidative enzyme.
MATERIAL AND METHODS
Addition and quantification of phenanthrene. A stock solution of FNN in acetone was prepared and sterilized by filtration. It was incorporated to 25 ml MS medium to have concentration of 0, 5, 15, 30 and 50 g/l (0, 28, 84, 168, 280 mM). Residual FNN was recovered by extraction with ethyl acetate/water (1:1) and estimated by HPLC analysis using a PRODIGY ODS2 column.
Reactions to study the EMS complex. Reactions were performed in 1.5 ml glass vials with 500μL of the reaction mixture (Potassium Buffer pH 6.0 (0.1 M), acetonitrile (1%), SDS 350 μM (1%), FNN (50 μM), QTN (300 μM), H2O2 (100 μM), radish peroxidase 0. 187 mU/μL), keeping this order for the addition of the reaction components. The reaction mixture was incubated by 24 hours at 37°C, and 180rpm on an orbital platform.
Extraction of metabolites from the EMS reactions. Extraction (3X) from the reaction mixture were performed with 750 μl of chloroform. The organic fraction was pulled and concentrated at 40°C, and resuspended to 1 ml with dimethylformamide:acetone (1:1). The aqueous phase (≈400 μL) was added with methanol (100 μL). Both fractions were analyzed by HPLC.
HPLC analysis. From the above extracts, 200 μL was taken for HPLC analysis. The aqueous fraction was resolved by using a prodigy ODS2 reverse phase column (C18, 25 cm x 4.6 mm; 5μm) attached to an HPLC UV/Visible equipment (Thermo Separation) as reported previously (Calva et al., 2016). However, to resolve the organic fraction, the gradient with trifluoroacetic acid 50μM (A) and acetonitrile (B) was: 0-4 min 0% B; 4-5 min 35% B; 5-8 min 35% B; 8-13 min 20% B, and 15-18 min 80% B. The spectral analysis was carried out by using the PC1000 and/or Chromeleon 7 softwre. Analytical compounds, including quercetin, quercitrin, chrysin, maysin, lutein, and rutin, were used as reference.
Statistical analysis. For the statistical analysis we stated the H2O2, the quercetin, and the FNN concentration as fixed factor. The SPSS V 15 statistical package (SPSS Inc., 2006) and the Microsoft Excel 2002 software were used for the statistical analysis and the estimation of the marginal means.
RESULTS AND DISCUSSION
Removal of phenanthrene and quercetin by the EMS system with quercetin. The removal ratio of phenanthrene (FNN) after 24 hours reaction, and quercetin (QTN) after 30 minutes reaction, by the EMS system, constituted by FNN 50µM (S), QTN 100 µM (M), H2O2 (100 μM), and 187 mU/μl of radish peroxidase (E), in comparison with several control reaction mixtures omitting one or several components of the EMS system, was studied (Figure 1).
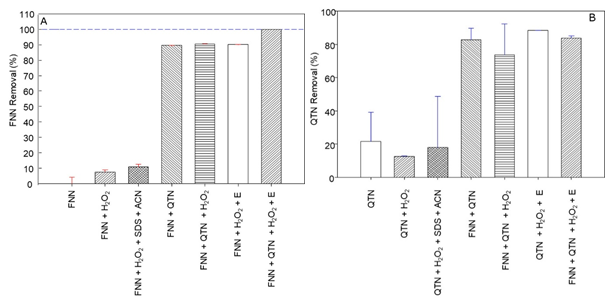
Fig. 1 Removal ratio of phenanthrene (FNN) after 24 hours reaction, and quercetin (QTN) after 30 minutes of incubation at 37 °C and 180 RPM, under the control reaction mixtures regarding to the complete EMS reaction mix constituted by phosphate buffer pH 6.0, FNN 50µM (S), QTN 100µM (M), and 187 mU/μl of radish peroxidase (POX). Acetonitrile (CAN), and sodium dodecyl sulfate (SDS) were used as adjuvants to dissolve the FNN.
Interestingly, in comparison with the complete EMS system (FNN+QTN+E+H2O2), which after 24 hours incubation, yielded 100% transformation FNN (Figure 1A), it was observed that in absence peroxidase (E), the presence of QTN itself or with H2O2, was able to promote ~92% transformation of FNN. A level similar to that produced when the E and H2O2 were present. Such observation agrees with previous hypothesis for the enzymatic removal of FNN (Calva et al., 2016), where the stoichiometric analysis predicted a removal of 100% of FNN (50µM) by the EMS system containing 300 µM QTN (Rivera-Casado et al., 2015a, Calva et al., 2016), and confirm the suggestion that the transformation of FNN does not require the participation of the oxidative E if QTN is present. This assumption matches with the removal ratio (Figure 1B), and profile (Figure 2) for the removal of QTN, which was completely removed earlier than FNN with a removal profile pattern very similar to that previously reported for FNN (Rivera-Casado et al., 2015a, Calva et al., 2016).
These results point out clearly that combination of QTN with H2O2 seems to be an important factor for the removal of FNN, and that H2O2 is used by peroxidases for the oxidation of both FNN and QTN. Therefore, the effect of H2O2 and the radish peroxidase over the oxidation of QTN in the EMS system was further examined and the results presented in the next section.
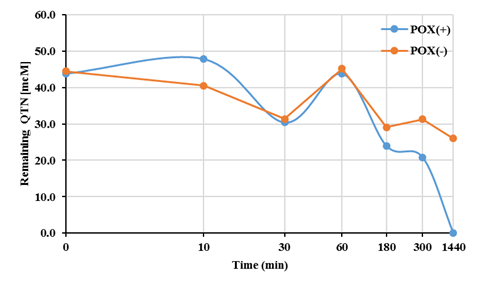
Fig. 2 Quercetin removal profile in the MES system for FNN oxidation using QTN as a natural mediator and 10 μM of H2O2 in the presence (+) and absence (-) of radish peroxidase (POX).
Effect of H2O2 and peroxidase over the oxidation of QTN in the EMS system. The removal of QTN as function of the H2O2 initial amount (0-30 mM) for the oxidation of FNN by the EMS reaction mixture after three incubation times (2, 30. 180 minutes), in the presence (EMS), and absence (MS), of radish peroxidase was examined by using a factorial 5x2 experimental design by estimation of the residual QTN (Figure 3). As expected, as the incubation time increases, the remanent QTN was lower, however the amount with (EMS) and without (EM9 peroxidase was similar. As mentioned above, the presence and concentration of H2O2 are fundamental parameters for the activity of peroxidases. The concentration range of H2O2 was chosen on the bases of reports for peroxidases from plant tissue, which corresponds to 0.6 - 10 μmol/g of fresh tissue (Cheeseman 2006).
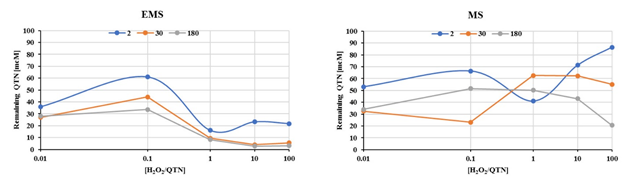
Fig. 3 Quercetin removal as function of the H2O2 amount throughout the oxidation of FNN in the EMS reaction mixture a three incubation times (2, 30. 180 minutes) in the presence (EMS), and absence (MS), of radish peroxidase. The assays were performed in 500μL reaction mixture containing 0.1M potassium buffer pH 6.0, 1% acetonitrile, 350μM SDS, 50 μM FNN (S), 300μM QTN (M), and 0.187 mU/μL radish peroxidase (E). Incubation time (0-1440 min) was performed at 37°C and 180rpm on an orbital shaker.
As can be appreciated, the results showed that oxidation of QTN occurs after 2 minutes from addition of the H2O2 to the reaction mixture, even in absence of the enzyme (Figure 3, MS). This observation was confirmed by HPLC spectral analysis for extracts from de reactions mix (Figure 4), where just after 3-5 minutes, the presence of QTN metabolites with retention times of 16.7 min, 24.92 min, and 27.7 min were clearly detected. Interestingly, an oxidation product with a retention time of 11.3 min was detected after 5 minutes of reaction, showing a spectrum that yield positive correlation with 3,4 dihydroxy-phenylglyoxylic acid, however no further identification of this compound was performed.
Even though the oxidation products of QTN by action of the EMS system were not identified with certainty, by comparing the absorption spectra, these results point out two important findings. First, the oxidation of QTN produce several unstable metabolites, probably by sequential reactions as suggested by Li et al. (2020). And second, peroxidase participation is not necessary for oxidation of QTN, which otherwise was clearly accelerated by H2O2. With these results, the reaction conditions and concentration of the EMS components were established to investigate the oxidation process of FNN.
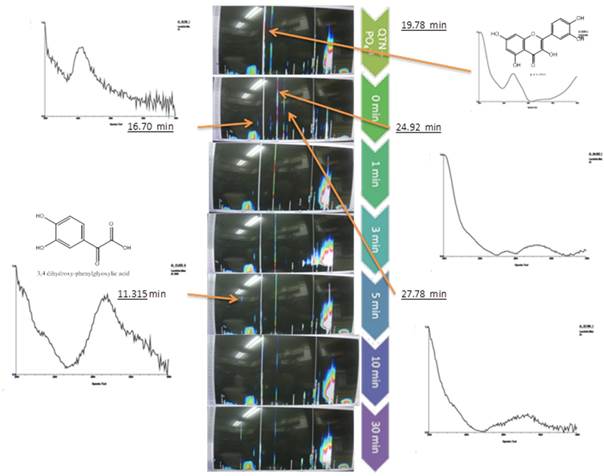
Fig. 4 Isochromatic graphs of the chromatogram and UV (center), and the spectral analysis of the QTN oxidation products in the EMS system with radish peroxidase at several reaction times compared to the non-enzyme reaction mixture (top chromatogram). Time zero was considered after adding the enzyme, stirring the reaction mixture and adding the solvent to stop the reaction.
Removal of FNN in the EMS system by using QTN as metabolic mediator. The FNN removal was up to 50% in 24h (Figure 5), with a rate of disappearance of 0.047 μM FNN/min during the first 5h. These results were similar to that reported previously for the kinetic for oxidation of FNN in the presence of QTN (Rivera-Casado 2010, Calva 2016); however, it is interesting to note that the behavior of the FNN removal curve after 6.7 hours was similar in the presence (POX+) and in the absence (POX-) of peroxidase (Figure 5). These observations confirm that the presence of the enzyme was not decisive for the removal of FNN, but only in very short reaction times, or that the enzyme catalyzes the transformation of other compound at early times. This hypothesis was supported by the fact that after 24 hours of reaction, the remaining amount of QTN was estimated to be 41%. It should be noted that this data is the first report where the variation of the concentration of the metabolic mediator through the removal of PAH by EMS system has been reported. Further, as observed in Table 1, the variation in the rate of transformation of FNN as a function of the concentration of H2O2, shows that the presence of H2O2 up to a concentration of 3000 μM, in general, positively affects the transformation of FNN, reaching values for rate of disappearance up to 0.02 μM FNN/min. However, a negative effect was observed at excessive concentrations of H2O2 (30000 μM). Interestingly, in the absence of enzyme (E-), the rate of disappearance of FNN at low peroxide concentrations showed values similar to the maximums reached with the complete reaction mixture, and gradually decreased with the increase in the amount of H2O2. However, in the absence of the QTN mediator (M-), the rate increased or remained almost constant at peroxide concentrations below 30000 μM.
Table 1 Rate of FNN removal as a function of H2O2 concentration in the MSE system with QTN as metabolic mediator.
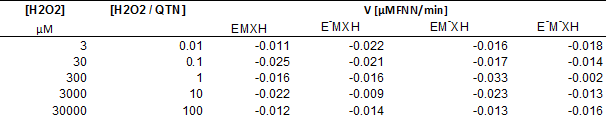
The complete reaction mix EMXH was constituted by FNN 50µM (X), QTN 100µM (M), and 187 mU/μl of radish peroxidase (E), while incomplete reactions mix lack of peroxidase enzyme (E-), QTN (M-), or peroxidase enzyme and QTN (E-M-XH).
The above results demonstrates that in the presence of small amounts of peroxide, probably at H2O2/QTN ratios less than 0.01, QTN could be partially oxidized to chemical forms capable of promoting FNN removal without the involvement of enzymes. This might suggest that in the complete mixture (EMSH), the E could use peroxide preferably to oxidize QTN, so in the absence of QTN the enzyme can direct its oxidative activity towards the FNN. In other words, in the experimental system used, the transformation of FNN depends on the presence of the mediator (QTN) or enzyme, not both. Thus, in congruence with opinions from the literature for similar systems (Yamasaki et al., 1997; Hernández et al., 2008), the results from this work suggest that H2O2 may act as a substrate for the enzyme or as generator of QTN free radicals by interacting with reactive oxygen species. The QTN free radicals can then interact chemically with FNN yielding the observed FNN-QTN complex observed in the EMS system and in the phenolic extracts previously reported (Rivera-Casado 2010, Calva 2016), and also detected in the EMS reactions (Figure 5B). Altogether, the results indicate that in the transformation of FNN under the presence of QTN as metabolic mediator, the participation of the peroxidase enzyme is not necessary, since only the presence of H2O2 can promote the oxidation of the metabolic mediator and this in turn the oxidation of the PAH.
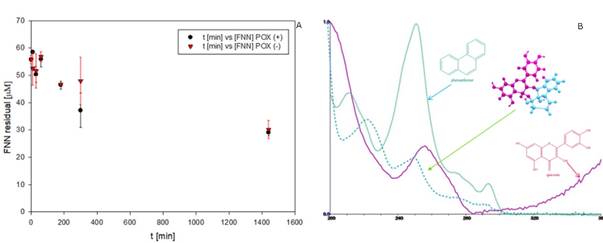
Fig. 5 Removal pattern of FNN in presence (+), and absence (-), of peroxidase (POX) in the EMS system (A), and UV spectrum of the putative QTN-FNN conjugate detected in the EMS reactions (B). The reaction mix was constituted by FNN 50µM, QTN 100µM (M), H202 10 µM, and 187 mU/μl of radish peroxidase.
CONCLUSIONS
These results with both quercetin and FNN revealed that peroxidase participation is not compulsory for the phenanthrene removal process, but that under these conditions the rate of transformation is regulated exclusively by the H2O2 concentration. The transformation products suggest that the removal process in the EMS system, with and without enzyme, was a sequential oxidation of the molecule mediated by quercetin and stimulated by the presence of H2O2 yielding QTN-FNN conjugates intermediates that might be furtherer transformed.














