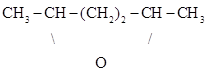INTRODUCTION
Life and career (Béhal, 1906; Vincent, 1941, Wikipedia, 2020, Anonymous, 2022)
Auguste Béhal (Figure 1) was born on March 30, 1859, in Lens (Pas-de Calais), the son of Auguste Béhal (1829-1907), at some time innkeeper, merchant, manufacturer of oil, and farmer, and Agnes Victorine Duquesne (1830-1926). He took his basic education in schools of the region and terminated it in 1875 with a second-class diploma with only a grammar certificate (certificat de grammaire), a fact that would latter affect his academic career because it allowed him only the possibility of graduating as pharmacien de 2e classe or health officer. In spit of this limitation, he decided to study pharmacy and carried on his apprenticeship in Lens, Lille and Paris. In 1878 he began his volunteer military work in Alençon, serving for one year in the 11th battalion of hunters on foot. The following year he enrolled in the École Supérieure de Pharmacie de Paris and prepared for the internship competition, which he won with the first place among more than forty candidates. He served at the Hôpital de la Pitié, where he became a close friend of Victor Grignard (1871-1935; 1912 Nobel Prize in Chemistry). His stage at the hospital was very successful, winning twice the silver medal of the hospitals (1882 and 1884) and the 1885 gold medal. During these years he prepared for the baccalauréat, which he obtained in 1883. In 1884, at the end of his studies, he was awarded exceptionally, to take the exams that allowed him to change his status from pharmacien de 2e classe to pharmacien de 1e classe. In the same year he entered the laboratory of Charles Friedel (1832-1899) at the Sorbonne and received his license ès sciences. While working for Friedel he completed his doctoral studies at the Faculty of Sciences of Paris, after successfully defending a thesis about acetylenic hydrocarbons (Béhal, 1888j). The members of the examining committee were Friedel, Henri Debray (1827-1888), and Gabriel Lippmann (1845-1921; 1908 Nobel Prize for Physics). The following year he won the aggregate competition (the door to an academic career) of the École Supérieure de Pharmacie with a thesis regarding azo compounds (Béhal, 1889b), and was appointed for five years adjunct professor at the École. During this period, he led the campaign for a change in the nomenclature of organic chemistry (Leclercq, 2007). As a result, he was appointed member of the French delegation to the 1892 international Geneva conference convoked for this purpose.
The period 1894-1906 saw the turbulent events of the Dreyfus affair where most of the French population supported the anti-Semitic and anti-Dreyfus campaign; Béhal did not join this campaign and backed the pro-Dreyfus group in favor of the innocence of the captain. This led to the termination of his appointment at the École Supérieure de Pharmacie and the potential end of an academic career. After a short position at the Bichat hospital (1886-1888) he was appointed chief pharmacist of the hospital of the Midi in Paris. In 1898 Friedel returned him to the academic world with an appointment at the Sorbonne as maître de conferences in organic chemistry. In 1901 he was appointed to the chair of toxicology at the École Supérieure de Pharmacie and in 1908 the École appointed him to the chair of organic chemistry, which he retained until his retirement in 1934, being then appointed honorary professor. Béhal also served as professor of elementary chemistry at the Union Française de la Jeunesse (1882-1886, 1888).
Béhal passed away on February 2, 1941, at his home in Mennecy (Essone), and was buried there.
Béhal served in many scientific and public positions, among them, chief pharmacist of the Port-Royal maternity (1904), member of the Société Chimique de France (1886) where he served as general secretary (1893 and 1905), vice-president (1908), president of the same (1922), and honorary president (1934); member of the Société de Pharmacie and its president (1905); president of the Franco-British exposition in London (1908), president of the Pure and Applied Chemistry Congresses held in Madrid (1934) and Rome (1938), president of the administration council of the Maison de la Chimie; member of the Académie de Medicine (1907) and its president in 1922; and member of the Académie des Sciences (1921) and its president in 1939. He was also member of the Société Thérapeutique. During the First World War he served as founder and first director of the Office des Produits Chimiques et Pharmaceutiques (1914-1918). The Académie des Sciences awarded him the 1891 and 1900 Jecker Prize (organic chemistry) and the 1894 Parkin Prize (medicine and surgery). The Société de Chimie Industrielle (afterwards Académie de Pharmacie) awarded him the 1933 Grand Gold Medal; Béhal was president of the same (1905). In 1896 he was elected Officier de l'Académie and in 1901 Officier de l'Instruction Publique (national French orders for distinguished academics); In 1901 Béhal was appointed chevalier de la Couronne Royale of Prussia and in 1904 he was nominated Chevalier de la Légion d'Honneur and promoted to Officier (1910), to Commandeur (1919), and to Grand Officier (1929).
Scientific contribution
Béhal wrote about 110 papers and books (i.e., Béhal, 1889b, 1890, 1893e, 1895c, 1897, 1926; Choay and Béhal, 1894) on the subjects of inorganic, organic, and biochemistry, physiology, etc. As customary for candidates to the Académie des Sciences and the Académie de Médicine, he published a booklet describing the results of his scientific research (Béhal, 1906). In addition to the subjects described below, he studied the separation of copper and cadmium (Béhal, 1885a); he described the new chemical nomenclature (Béhal, 1892); he studied camphor and derivatives (Béhal, 1894a b, 1895a b);16-19 malonic acid and derivatives (Béhal and Auger, 1888a b, 1892); and chloroform (Béhal and François, 1897); he determined that quinone was the active principle of the venom of the millipede Julus terrestris (Béhal and Phisalix, 1900); he studied the preparation and properties of the mixed anhydrides of acyclic and cyclic acids (Béhal, 1900); the isolation and structure of anethole and its isomer (Béhal and Tiffeneau, 1901); etc. Béhal's contribution to chemical nomenclature and atomic theory will not be presented here; they have been discussed in other publications (Delépine, 1960; Leclercq, 2007).
Acetylenic hydrocarbons
Béhal wrote that at the time he had started to study the acetylenic hydrocarbons (1883), they included a wide variety of compounds, among them acetylene, crotonylene (2-butyne), diallyl, etc. (Béhal, 1906). Béhal decided to try to find if the hydration of these compounds yielded a solid base for their classification. In his first paper he reported the hydration of œnanthylidene (1-heptyne), the first acetylenic hydrocarbon to have a well-known formula (Béhal, 1885b, 1888b). It was prepared by the method reported by Emil Rubien, starting from œnanthaldehyde (Rubien, 1867): Treatment of œnanthol with phosphorus pentachloride yielded œnanthylene chloride, C7H14Cl2, which treated with alcoholic KOH transformed into chlorinated œnanthylene, C7H13Cl. Further reaction with KOH in a closed tube at 140o-150 oC led to the desired 1-heptyne. Béhal treated the hydrocarbon with diluted sulfuric acid and distilled the solution in the presence of an air stream to avoid violent jolts. Analysis of the fraction passing at 147o-148 oC (763 mmHg) indicated that it contained, by weight, 73.26% carbon, 12.19% hydrogen, and 14.55% oxygen; this material was found to be a ketone, which did not react with an ammonia solution of silver nitrate or with the Fehling liquor. Under the action of strong oxidants (i.e., chromic mixture) it yielded valeric acid, C5H10O2 (a mixture of the normal and isovaleric acids) (Béhal, 1885b, 1888b).
The following publication discussed the hydration of caprylidene, which Béhal prepared by treating caprylic aldehyde (octanal), obtained from the dry distillation of the neutral soap of castor oil, with phosphorus pentachloride: C8H16O + PCl5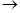 POCl2 + C8H16Cl2 (Béhal, 1887a, 1888b). The resulting 1-octene chloride was treated with boiling KOH during 72 hours in a flask provided with reflux, yielding chlorinated octene, C8H15Cl, and a little of octylidene. The chlorinated octene was converted to caprylidene by heating it with KOH at 150 oC in a closed tube for 12 hours, and the caprylidene hydrated by adding it drop-wise to sulfuric acid cooled with ice. The product was purified by distillation, the pertinent fraction passed at 171 oC (761.7 mmHg). Elemental analysis indicated that its composition corresponded to the formula C8H16O. The reaction that produced this ketone was very simple: a sulfoconjugated acid was formed that was decomposed by water regenerating the sulfuric acid and liberating the hydrate of the acetylenic hydrocarbon. This ketone was a colorless and very fluid liquid, smelling like an apple, having a burning aromatic taste, and relative density 0.8351 (0 oC). It was soluble in alcohol and ether and insoluble in water and did not react with an ammonia solution of silver nitrate or with the Fehling liquor. It combined with sodium bisulfite and the product was decomposed by hot water. The chromic mixture decomposed it into caproic and acetic acids. According to Béhal, caprylidene was a true acetylenic hydrocarbon, it produced with an ammonia solution of cuprous chloride or silver nitrate the precipitates characteristic of these hydrocarbons. It was a very mobile liquid, with a strong smell, relative density 0.7711 (0 oC), and boiling between 133o and 134 oC. Caprylic aldehyde reacted with phosphorus pentachloride to yield caprylene chloride that released HCl and generated monochloro caprylene, boiling at 167o-168 oC and having relative density 0.9274. He also showed that caprylic aldehyde was substantially different from methylhexyl ketone (2-octanone), although they had similar physical properties. Methylhexyl ketone did not react with an ammonia solution of silver nitrate or with the Fehling liquor, even after prolonged boiling. In addition, the oxidation of caprylic aldehyde by hot oxygen yielded caprylic acid (Béhal, 1887a, 1888b). A following paper gave more information about the differences between these two substances, based on their reaction with hydroxylamine. It was known that hydroxylamine reacted with aldehydes and ketones eliminating one molecule of water and yielding isomeric nitrogenated products. Béhal found that that the resulting aldoxime and ketoxime boiled at 121o-123 oC and 116o-117 oC, respectively (Béhal, 1887b, 1888b).
POCl2 + C8H16Cl2 (Béhal, 1887a, 1888b). The resulting 1-octene chloride was treated with boiling KOH during 72 hours in a flask provided with reflux, yielding chlorinated octene, C8H15Cl, and a little of octylidene. The chlorinated octene was converted to caprylidene by heating it with KOH at 150 oC in a closed tube for 12 hours, and the caprylidene hydrated by adding it drop-wise to sulfuric acid cooled with ice. The product was purified by distillation, the pertinent fraction passed at 171 oC (761.7 mmHg). Elemental analysis indicated that its composition corresponded to the formula C8H16O. The reaction that produced this ketone was very simple: a sulfoconjugated acid was formed that was decomposed by water regenerating the sulfuric acid and liberating the hydrate of the acetylenic hydrocarbon. This ketone was a colorless and very fluid liquid, smelling like an apple, having a burning aromatic taste, and relative density 0.8351 (0 oC). It was soluble in alcohol and ether and insoluble in water and did not react with an ammonia solution of silver nitrate or with the Fehling liquor. It combined with sodium bisulfite and the product was decomposed by hot water. The chromic mixture decomposed it into caproic and acetic acids. According to Béhal, caprylidene was a true acetylenic hydrocarbon, it produced with an ammonia solution of cuprous chloride or silver nitrate the precipitates characteristic of these hydrocarbons. It was a very mobile liquid, with a strong smell, relative density 0.7711 (0 oC), and boiling between 133o and 134 oC. Caprylic aldehyde reacted with phosphorus pentachloride to yield caprylene chloride that released HCl and generated monochloro caprylene, boiling at 167o-168 oC and having relative density 0.9274. He also showed that caprylic aldehyde was substantially different from methylhexyl ketone (2-octanone), although they had similar physical properties. Methylhexyl ketone did not react with an ammonia solution of silver nitrate or with the Fehling liquor, even after prolonged boiling. In addition, the oxidation of caprylic aldehyde by hot oxygen yielded caprylic acid (Béhal, 1887a, 1888b). A following paper gave more information about the differences between these two substances, based on their reaction with hydroxylamine. It was known that hydroxylamine reacted with aldehydes and ketones eliminating one molecule of water and yielding isomeric nitrogenated products. Béhal found that that the resulting aldoxime and ketoxime boiled at 121o-123 oC and 116o-117 oC, respectively (Béhal, 1887b, 1888b).
The next paper discussed the preparation of allyl iodide and allyl alcohol (Béhal, 1887c, 1888b). Béhal wrote that three procedures were available for the preparation of allyl iodide. The first one consisted in reacting glycerin with phosphorus iodide or red phosphorus (Berthelot & de Luca, 1856); this procedure was inconvenient because it operated only on very small amounts of glycerin and required the use of a large flask due to the abundant amount of mousse formed. The second method consisted in reacting little by little white phosphorus with glycerin (Saytzeff & Kanonnikoff, 1877). This permitted production of large amounts of the product but the need of using a stream of CO2 and a large flask (to avoid explosions) made it unpractical. The third procedure consisted in reacting allyl alcohol with phosphorus iodide or with red phosphorus and iodine (Cahours & Hofmann, 1856). This procedure had the inconvenience of requiring a very large time for obtaining dry and pure allyl alcohol but had the advantage of not producing isopropyl iodide when operating with a large excess of allyl alcohol. Béhal modified the Berthelot and de Luca process as follows: a mixture of 200 g of commercial glycerin, 600 g of iodine, and 200 g of red phosphorus was introduced into a 4-liter flask provided with reflux and heated under agitation until it achieved the consistency of mousse. Afterwards, a solution of 400 g of iodine in allyl iodide was added dropwise. The aqueous liquid that distilled contained a large proportion of allyl alcohol. Treated with sodium carbonate it separated a very mobile liquid that distilled at 90 oC. This new liquid was treated with quicklime and found to distill a 103 oC. The overall yield was about 100 g of allyl alcohol. The lower layer contained allyl iodine mixed with a little of allyl alcohol; the iodide was found to distill at 101 oC (Béhal, 1887c, 1888b).
Béhal also studied the hydration of diallyl, an acetylenic hydrocarbon, CH2=CH-CH2-CH2-CH=CH2, prepared by the method of Berthelot and de Luca (Berthelot & de Luca, 1855; Béhal, 1887d, 1888b). In theory, the hydration of this substance by means of sulfuric acid should produce diethylene glycol, CH3-CHOH-CH2-CH2-CHOH-CH3. Nevertheless, Carl Schorlemmer had reported the formation of polymers boiling above 200 oC (Schorlemmer, 1866), and W. R. Jekyll the formation of a diallylene oxide (Jekyll, 1871). Jekyll dissolved diallyl in paraffin oil (boiling at 55o-60 oC) and then added sulfuric acid dropwise until no more reaction was observed. At the end of the reaction, he obtained two liquid phases. Treatment of the lower phase with water separated a strongly colored light oil, which was distilled and then subjected to the same procedure. The new light oil was dried over calcium chloride and potassium. The dried material boiled at 93 oC and its composition corresponded to the formula C6H12O. According to Jekyll, this was the substance that Charles Adolph Würtz (1817-1884) had described under the name diallyl monohydrate or pseudo hexylene oxide (Jekyll, 1871; Würtz, 1864). It was soluble in sulfuric acid, its oxidation yielded acetic and CO2, it was not attacked by the sodium amalgam and hydrogen hydride transformed it into hexyl ( iodide boiling at 165o-167 oC (Jekyll, 1871).
Béhal employed a diallyl boiling at 58o to 60 oC, which he hydrated by drop-wise addition to sulfuric acid cooled with ice (Béhal, 1887d, 1888b). The resulting mixture was neutralized with an alkaline base in the presence of ice and then distilled. The upper layer of the distillate was the oxide, boiling at 93 oC, soluble in water, and not reacting with sodium bisulfite, hydroxylamine hydrochloride, an ammonia solution of silver nitrate, and with magnesium chloride. Béhal submitted the product to chemical tests with a variety of reagents and concluded that the hydration of diallyl by means of sulfuric acid produced the following derivatives: (1) an oxide originating from the dehydration of isohexyl glycol (dimethylbutyl glycol), identical with the pseudo hexylene oxide of Würtz, and having the following formula:
(2) A sulfoconjugated acid containing only one molecule of sulfuric acid per two ethylene functions; and (3) an abundant amount of diallyl polymers (Béhal, 1887d, 1888b).
Béhal also prepared ethyl propyl acetylene, a new substituted acetylenic hydrocarbon, by reacting piecewise butyrone (dipropyl ketone), boiling at 142o to 146 oC, with phosphorus pentachloride (Béhal, 1887e, 1888c). This was a very energetic reaction, accompanied by release of HCl. The decanted product was boiled with reflux with an alcoholic solution of KOH for 24 hours, and then treated with water. The resulting supernatant liquid was separated, dried with calcium chloride, and distilled. The process was repeated several times until producing a liquid boiling at 105o to 106 oC, relative density 0.769 (0 oC), and containing, by weight, 86.65% carbon and 12.49% hydrogen, corresponding to ethyl propyl acetylene, 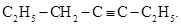 This new hydrocarbon did not combine with ammonia cuprous chloride but gave a white product with mercuric chloride. It combined energetically with bromine. Hydration with sulfuric acid resulted in the regeneration of butyrone (Béhal, 1887e, 1888c).
This new hydrocarbon did not combine with ammonia cuprous chloride but gave a white product with mercuric chloride. It combined energetically with bromine. Hydration with sulfuric acid resulted in the regeneration of butyrone (Béhal, 1887e, 1888c).
Béhal also described the preparation of caprylene (1-octene) caprylidene, 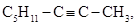 an isomer of the caprylic aldehyde caprylidene he had reported previously (Béhal, 1887a
f, 1888b). This compound had previously been prepared by Heinrich Limpricht (1827-1909) and Rubien (Rubien, 1867). Béhal prepared caprylene by dehydrating caprylic alcohol with zinc chloride. This substance did not react with ammonia cuprous chloride or silver nitrate (indicating that it was a substituted acetylenic compound) but reacted with an aqueous solution of mercuric chloride. Béhal carried on the hydration by the same procedure (sulfuric acid) used for its isomer (Béhal, 1887a
f, 1888b).
an isomer of the caprylic aldehyde caprylidene he had reported previously (Béhal, 1887a
f, 1888b). This compound had previously been prepared by Heinrich Limpricht (1827-1909) and Rubien (Rubien, 1867). Béhal prepared caprylene by dehydrating caprylic alcohol with zinc chloride. This substance did not react with ammonia cuprous chloride or silver nitrate (indicating that it was a substituted acetylenic compound) but reacted with an aqueous solution of mercuric chloride. Béhal carried on the hydration by the same procedure (sulfuric acid) used for its isomer (Béhal, 1887a
f, 1888b).
Béhal tried unsuccessfully to prepare gaseous allene (propadiene) by a variety of procedures, among them, from allyl iodide, trimethylene bromide, allyl alcohol, ethyl allyl ether, diallyl ether, ( and ( epichlorhydrin, and electrolysis of itaconic acid (Béhal, 1887g).
He also reported the preparation of isopropyl acetylene from methyl isopropyl ketone, (CH3)2CH-CO-CH3. This ketone was prepared by reacting isobutyric chloride with methyl zinc (Béhal, 1888a c, 1889a).
Béhal summarized all the above information in two publications, giving a more detailed description of the operating methods and the properties of the different derivatives prepared (Béhal, 1888b
c). He used his results to classify the quadrivalent hydrocarbons into four categories: (1) mono substituted acetylenic hydrocarbons, of formula 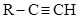 (for acetylene R = H and for the other members, R = CnH2n+1). These were the true acetylenic hydrocarbons because they precipitated an ammonia solution of cuprous chloride or silver nitrate; (2) disubstituted acetylenic hydrocarbons, of formula
(for acetylene R = H and for the other members, R = CnH2n+1). These were the true acetylenic hydrocarbons because they precipitated an ammonia solution of cuprous chloride or silver nitrate; (2) disubstituted acetylenic hydrocarbons, of formula 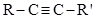 (where R' could be identical with R), which did not react with the above reagents; (3) diethylene hydrocarbons, of formula R-CH=CH-CH=CH-R, and (4) allyl hydrocarbons of formula
(where R' could be identical with R), which did not react with the above reagents; (3) diethylene hydrocarbons, of formula R-CH=CH-CH=CH-R, and (4) allyl hydrocarbons of formula 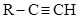 (Béhal, 1888b). Béhal hydrated the hydrocarbons by two procedures, the first one due to Michael Kutcheroff and applicable to true or substituted acetylenic hydrocarbons (Kutcheroff, 1884). It consisted in combining the hydrocarbon with mercuric chloride, sulfate, or acetate and decomposing the resulting hydrate. Applied to acetylene, allylene (methyl acetylene, 1-propyne), ethyl acetylene (1-butyne), and ethyl methyl acetylene (pent-2-yne) it yielded acetaldehyde with acetylene, and methyl ketones with the other compounds. The second procedure was applicable to all tetratomic hydrocarbons and consisted in reacting the hydrocarbon with sulfuric acid and decomposing the sulfoconjugated derivative with an excess of water (Berthelot, 1862). Béhal was the first to test it on acetylene, allylene, isopropyl acetylene, valerylene, and diallyl (Béhal, 1888b).
(Béhal, 1888b). Béhal hydrated the hydrocarbons by two procedures, the first one due to Michael Kutcheroff and applicable to true or substituted acetylenic hydrocarbons (Kutcheroff, 1884). It consisted in combining the hydrocarbon with mercuric chloride, sulfate, or acetate and decomposing the resulting hydrate. Applied to acetylene, allylene (methyl acetylene, 1-propyne), ethyl acetylene (1-butyne), and ethyl methyl acetylene (pent-2-yne) it yielded acetaldehyde with acetylene, and methyl ketones with the other compounds. The second procedure was applicable to all tetratomic hydrocarbons and consisted in reacting the hydrocarbon with sulfuric acid and decomposing the sulfoconjugated derivative with an excess of water (Berthelot, 1862). Béhal was the first to test it on acetylene, allylene, isopropyl acetylene, valerylene, and diallyl (Béhal, 1888b).
The second paper Béhal discussed the situation with substituted acetylenic hydrocarbons, represented by the formula 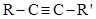 (Béhal, 1888c). The number of known hydrocarbons of this nature was restricted and, in several cases, it was not sure that their constitution had been properly identified, for example, dimethyl acetylene, valerylene, methyl ethyl acetylene, the hydrocarbon derived from butyrone, ethyl propyl acetylene, methyl valeryl acetylene, a hydrocarbon produced by the reaction between hexylene bromide (from mannitol) and alcoholic KOH, hexoylene, heptolylene, octoylene, and nonoylene. Béhal wrote that this type of hydrocarbon was prepared by three methods. The first procedure was based on the reaction between phosphorus pentachloride and a dichloride derivative of a ketone (where the two constituent groups were at least ethyl), which had been treated with alcoholic KOH. Thus, diethyl ketone generated methyl ethyl acetylene, and dipropyl ketone, ethyl propyl acetylene. A ketone of formula (R)2CH-CO-CH(R)2 would yield an allylic hydrocarbon while ketones of the structures (R)3C-CO-CH(R)2 and (R)3C-CO-C(R)3 would not react. The second method started by preparing the dibromo derivative of a non-terminal ethylenic hydrocarbon followed by abstraction of two molecules of HBr by means of alcoholic KOH. The third method was based on the transformation of true acetylenic hydrocarbons into substituted acetylenic by the migration of the acetylenic functional group, under the influence of alcoholic KOH (Béhal, 1888c).
(Béhal, 1888c). The number of known hydrocarbons of this nature was restricted and, in several cases, it was not sure that their constitution had been properly identified, for example, dimethyl acetylene, valerylene, methyl ethyl acetylene, the hydrocarbon derived from butyrone, ethyl propyl acetylene, methyl valeryl acetylene, a hydrocarbon produced by the reaction between hexylene bromide (from mannitol) and alcoholic KOH, hexoylene, heptolylene, octoylene, and nonoylene. Béhal wrote that this type of hydrocarbon was prepared by three methods. The first procedure was based on the reaction between phosphorus pentachloride and a dichloride derivative of a ketone (where the two constituent groups were at least ethyl), which had been treated with alcoholic KOH. Thus, diethyl ketone generated methyl ethyl acetylene, and dipropyl ketone, ethyl propyl acetylene. A ketone of formula (R)2CH-CO-CH(R)2 would yield an allylic hydrocarbon while ketones of the structures (R)3C-CO-CH(R)2 and (R)3C-CO-C(R)3 would not react. The second method started by preparing the dibromo derivative of a non-terminal ethylenic hydrocarbon followed by abstraction of two molecules of HBr by means of alcoholic KOH. The third method was based on the transformation of true acetylenic hydrocarbons into substituted acetylenic by the migration of the acetylenic functional group, under the influence of alcoholic KOH (Béhal, 1888c).
Very few of these hydrocarbons had been hydrated. For example, the hydration of dimethyl acetylene yielded hexamethylbenzene (mellitene) accompanied by a little of methyl ethyl ketone; the hydration of methyl ethyl acetylene with mercuric bromide produced methyl propyl ketone, and with sulfuric acid a hydrate of divalerylene (dipentine), trivalerylene, and a polymer. Béhal had reported the hydration of caprylene caprylidene (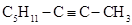 ), ethyl propyl acetylene, and a new heptylidene. In this new publication he added the results of the hydration of tolane (diphenyl acetylene), which he obtained by treating stilbene bromide with alcoholic KOH, as reported by Limpricht and H. Schwanert (Limpricht & Schwanert, 1871). Tolane did not combine with an aqueous or alcoholic solution of mercuric chloride. The hydration was carried with sulfuric acid. The resulting phenyl benzyl ketone appeared as white crystals melting at 54o-55 oC and containing, by weight. 85.56% carbon, 6.50% hydrogen, and 7.94% oxygen (Béhal, 1888c
e).
), ethyl propyl acetylene, and a new heptylidene. In this new publication he added the results of the hydration of tolane (diphenyl acetylene), which he obtained by treating stilbene bromide with alcoholic KOH, as reported by Limpricht and H. Schwanert (Limpricht & Schwanert, 1871). Tolane did not combine with an aqueous or alcoholic solution of mercuric chloride. The hydration was carried with sulfuric acid. The resulting phenyl benzyl ketone appeared as white crystals melting at 54o-55 oC and containing, by weight. 85.56% carbon, 6.50% hydrogen, and 7.94% oxygen (Béhal, 1888c
e).
Béhal wrote that one of the usual methods for identifying an acetylenic hydrocarbon was to contact it with an ammonia solution of cuprous chloride or of silver nitrate. These methods were highly sensitive for gaseous compounds or liquid compounds of relatively low molecular weight, but not so for compounds of high molecular weight, such as œnanthylidene (heptyne). This led Béhal to investigate the possibility of using other reagents as the identifying agent for this type of hydrocarbons. His results indicated that a saturated alcoholic solution of silver nitrate was very appropriate; the reagent produced a white crystalline precipitate with pure acetylenic compounds, or a mixture of them, but did not react with substituted acetylenic hydrocarbons (Béhal, 1888c d).
Béhal also investigated the transformation of œnanthylidene and caprylidene (true acetylenic hydrocarbons) into substituted acetylenic hydrocarbons, under the influence of alcoholic KOH (Béhal, 1888c
f), the use of sodium to convert non-acetylenic caprylidene into an acetylenic hydrocarbon (Béhal, 1888c
g), and methyl valeryl acetylene into hexyl acetylene (Béhal, 1888c
h), and the hydration of methyl amyl acetylene 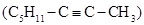 into ethyl amyl ketone, a new ketone (Béhal, 1888c
i).
into ethyl amyl ketone, a new ketone (Béhal, 1888c
i).
As mentioned before, all the above publications were the basis of Béhal’s doctoral thesis (Béhal, 1888j).
Béhal and Alexandre Desgrez (1863-1940) studied the action of organic acids on acetylenic hydrocarbons, (Béhal & Desgrez, 1892b). Their results indicated that heating at high temperature acetylenic hydrocarbons with acetic acid resulted in the formation an unstable combination, which in the presence of water transformed in ketones. The reactions were carried in sealed tubes; it began at 250 oC but took place mostly at 280 oC. Their study was based on two true acetylenic hydrocarbons, œnanthylidene and caprylidene, and two substituted acetylenic compounds, butyl methyl acetylene (2,3-heptyne), and pentyl methyl acetylene (2,3-octyne).
Œnanthylidene was obtained by the repeated action of dry KOH and phosphorus pentachloride on œnanthylic aldehyde. The resulting liquid was distilled under vacuum and the fraction distilling above 120 oC collected and mixed with water. The upper phase was steam distilled and the passing phase treated with sodium bisulfite. The bisulfite combination formed decomposed yielding a liquid boiling at 149o-150 oC (755 mmHg) and containing a ketone having, by weight, 72.73% carbon, 12.62% hydrogen, and 14.65% oxygen, corresponding to the formula C7H14O, that is, 2-heptanone. The three other acetylenic hydrocarbons were subjected to the same process. Caprylidene, obtained by the reaction between hexyl methyl acetone, KOH dry, and phosphorus pentachloride, yielded 2-octanone, boiling at 171 oC (758 mmHg). Butyl methyl acetylene, obtained by the molecular transposition of 1,2-heptyne, yielded 2-heptanone boiling between 149o-150 oC. Pentyl methyl acetylene, obtained from the reaction between caprylene bromide and dry KOH, yielded 2-octanone, boiling at 171 oC (Béhal & Desgrez, 1892).
Béhal and Desgrez also found that ethylenic hydrocarbons such as caprylene and allyl acetate, reacted with fatty acids (acetic acid) to yield products like caprylene acetate and propyl glycol diacetate (Béhal & Desgrez, 1892a).
Chloral and derivatives
Béhal and Eugène Choay (1861-1942) carried initially this work in order to study the preparation of ammonia chloral, CCl2-CH=NH, and its decomposition with time and temperature (Béhal & Choay, 1892a
b). In 1871 Jacques Personne (1816-1880) announced that under the influence of boiling water, chloral ammonia (1-amino-2,2,2-trichlorethanol) decomposed into chloroform and ammonium formate, according to CCl3-CH(NH2)(OH) + H2O 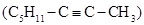 HCCl3 + HCOONH4. It was clear that in the absence of water the decomposition would be into formamide and chloroform CCl3-CH(NH2)(OH)
HCCl3 + HCOONH4. It was clear that in the absence of water the decomposition would be into formamide and chloroform CCl3-CH(NH2)(OH) 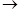 HCCl3 + HCONH2 (Personne, 1871).
HCCl3 + HCONH2 (Personne, 1871).
Béhal and Choay studied the decomposition of chloral ammonia at 100 oC and found that although most of the product was composed of formamide and chloroform, there was also formation of chloralimide, an isomer of it, and chloralformamide. This result led Béhal and Choay to study in detail chloralimide and its isomer, and the possibility that chloral might combine with substances containing nitrogen. Their results indicated that chloral hydrate was able to form two new compounds with phenyl dimethyl pyrazolone, of which one of them, hypnal, had found use in therapeutics (Béhal & Choay, 1892a; Behal, 1906).
According to Béhal and Choay, determination of the molecular mass of chloralimide by cryoscopy indicated that it should not be considered as CCl2-CH=NH but as its trimer, (CCl2-CH=NH)3, as was actually substantiated by its chemical reactions. Not only that, the isomer isolated from the residue of the decomposition reaction was also a trimer that under the influence of aqueous acids decomposed into chloral and the pertinent ammonia salt of the acid. These results showed that in this trimer the three molecules were united through the nitrogen atom. Béhal and Choay found that heating chloral ammonia in a flask provided with a condenser, over a bath of boiling water, resulted in the passing of chloroform and water charged with ammonia, and the residue becoming thicker and yellow while retaining with tenacity a certain amount of chloroform. Elimination of this chloroform could only be achieved by heating continuously at 100 oC, for a long period of time. The residue was then diluted with concentrated alcohol and the resulting solution eliminated under vacuum. The remaining ammonium chloride was eliminated by water washes. The purified residue was then dissolved in a large amount of alcohol and left to concentrate and crystalize by natural evaporation. The resulting long needles of chloralimide were dissolved in a mixture of absolute alcohol and recrystallized. Elemental analysis indicated that its composition corresponded to the formula C2Cl3NH2. The resulting residue was found to be isochloralimide (Béhal & Choay, 1892a).
Since the formation of chloralimide could be considered as the simple dehydration of chloral ammonia, CCl2-CH=NH 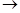 H2O + CCl3-CH=NH, Béhal and Choay decided to try to improve the yield of chloralimide by using dehydrating agents, such as zinc chloride, fused calcium chloride, fused calcium chloride in the presence of calcium carbonate, and anhydrous chloride The results indicated that the last agent was the best one. It turned out that addition of anhydrous chloride to chloral ammonia resulted in a very exothermic reaction, with the resulting increase in temperature and yield. Chloralimide was found to be insoluble in water and soluble in concentrated alcohol, benzene, chloroform, and acetic acid. It melted poorly at about 150o-155 oC and never gave place to a limpid liquid, particularly because the fusion was accompanied by the release of pure chlorine. Heated with water in a sealed tube it decomposed completely at 170o to 180 oC. It was rapidly attacked by cold dilute mineral acids and instantly by hot acids, yielding chloral, trichloroacetic acid, and the corresponding ammonium salt. A solution of platinum chloride split it into ammonium chloroplatinate and chloral, which combined with the alcohol to form chloral ethylate. It was very slowly attacked by a cold aqueous solution of potassium permanganate and complete destroyed by a hot solution of the salt. Chloralimide was attacked by a cold solution of bromine in chloroform, the reaction was very lively, with release of HBr and formation of two derivatives of chloralimide; one melted at 1050-106 oC and the other at 1550-156 oC. After purification, these compounds were found to be (-didehydrochloralimide and (-didehydrochloralimide, respectively. (-Didehydrochloralimide crystallized as clinorhombic prisms melting at 157 oC, it was insoluble in water and very soluble in alcohol, benzene, chloroform, and acetic acid. Béhal and Choay also described the preparation and properties of two other derivatives of chloralimide: oxyditrichloro-ethylidenediamine and ditrichloroethylenediamine ditrichloro acetylated. They also described the properties and the reaction of chloralimide with benzoyl chloride, the properties of isochloralimide and its reaction with acids, benzoyl chloride, alkalis, methyl iodide, the properties of didehydrochloralimide and its reaction with platinum chloride, an alcoholic solution of HCl, and aqueous mineral acids. They also gave a detailed crystallographic description of several of the crystallized materials they had synthesized, among them, chloralimide, isochloralimide, (-didehydrochloralimide, (-didehydrochloralimide, and oxyditrichloro-ethylidenediamine (Béhal & Choay, 1892a).
H2O + CCl3-CH=NH, Béhal and Choay decided to try to improve the yield of chloralimide by using dehydrating agents, such as zinc chloride, fused calcium chloride, fused calcium chloride in the presence of calcium carbonate, and anhydrous chloride The results indicated that the last agent was the best one. It turned out that addition of anhydrous chloride to chloral ammonia resulted in a very exothermic reaction, with the resulting increase in temperature and yield. Chloralimide was found to be insoluble in water and soluble in concentrated alcohol, benzene, chloroform, and acetic acid. It melted poorly at about 150o-155 oC and never gave place to a limpid liquid, particularly because the fusion was accompanied by the release of pure chlorine. Heated with water in a sealed tube it decomposed completely at 170o to 180 oC. It was rapidly attacked by cold dilute mineral acids and instantly by hot acids, yielding chloral, trichloroacetic acid, and the corresponding ammonium salt. A solution of platinum chloride split it into ammonium chloroplatinate and chloral, which combined with the alcohol to form chloral ethylate. It was very slowly attacked by a cold aqueous solution of potassium permanganate and complete destroyed by a hot solution of the salt. Chloralimide was attacked by a cold solution of bromine in chloroform, the reaction was very lively, with release of HBr and formation of two derivatives of chloralimide; one melted at 1050-106 oC and the other at 1550-156 oC. After purification, these compounds were found to be (-didehydrochloralimide and (-didehydrochloralimide, respectively. (-Didehydrochloralimide crystallized as clinorhombic prisms melting at 157 oC, it was insoluble in water and very soluble in alcohol, benzene, chloroform, and acetic acid. Béhal and Choay also described the preparation and properties of two other derivatives of chloralimide: oxyditrichloro-ethylidenediamine and ditrichloroethylenediamine ditrichloro acetylated. They also described the properties and the reaction of chloralimide with benzoyl chloride, the properties of isochloralimide and its reaction with acids, benzoyl chloride, alkalis, methyl iodide, the properties of didehydrochloralimide and its reaction with platinum chloride, an alcoholic solution of HCl, and aqueous mineral acids. They also gave a detailed crystallographic description of several of the crystallized materials they had synthesized, among them, chloralimide, isochloralimide, (-didehydrochloralimide, (-didehydrochloralimide, and oxyditrichloro-ethylidenediamine (Béhal & Choay, 1892a).
The second paper was a study of the decomposition of chloral ammonia at room temperature and of the byproduct chloral diformamide (Béhal & Choay, 1892b). The chloral ammonia obtained by the action of gaseous ammonia on chloral dissolved in chloroform, was purified by recrystallization in chloroform and dried under vacuum. The resulting crystalline powder decomposed very slowly, it softened first and then became liquid and separated a light phase smelling strong as ammonia, and then forming a crystalline deposit. The lower phase was concentrated under vacuum and the resulting crystals separated and then washed with alcohol and water. The resulting white residue was later shown to be chloral diformamide. Chloral diformamide was sparingly soluble in cold water and very soluble in hot absolute alcohol and in ether. It melted at 216o-217 oC and then decomposed, releasing ammonia and leaving a black residue. Elemental analysis indicated that it contained, by weight, 21.86% carbon, 2.27% hydrogen, 48.71% chlorine, and 12.73% of nitrogen, corresponding to the formula C4H5Cl3N2O2, the structure CCl3-CH=(NH-COH)2, and molecular mass 219.5. An alcoholic solution, heated with platinum chloride, yielded a precipitate of ammonium chloroplatinate (Béhal & Choay, 1892b).
Béhal and Choay reacted chloral diformamide with several reagents to try to obtain a better picture of its composition. Heating this substance with a concentrated solution of HCl it decomposed into chloral, ammonium chloride, ethyl formate, and CO. These and other exams suggested that the decomposition of chloral ammonia, at room temperature, went through the following stages:
(1) Two molecules of chloral ammonia split into chloroform and formamide:
2CCl3-CH(OH)(NH2) 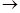 2CHCl3 + 2H-CO-NH2
2CHCl3 + 2H-CO-NH2
(2) The formamide formed reacted with one molecule of chloral ammonia yielding water, ammonia, and chloral diformamide:
CCl3-CH(OH)(NH2) + 2HCO-NH2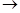 H2O + NH3 + CCl3-CH(NH-COH)2
H2O + NH3 + CCl3-CH(NH-COH)2
Ammonium chloride was formed by a secondary reaction between ammonia and the chlorine in the pertinent derivatives (Béhal & Choay, 1892b).
Béhal and Choay studied in detail the reaction between chloral and phenyl methyl pyrazolone. As mentioned before, this reaction produced two clearly defined substances, one containing one molecule of chloral and one molecule of pyrazolone, and the other, two molecules of chloral and one of pyrazolone, which Béhal and Choay named monochloro antipyrine and dichloro antipyrine, respectively. Monochloro antipyrine melted at 67o-68 oC and was soluble in water. Its aqueous solution reacted with ferric chloride and assumed a red color similar to the one produced by antipyrine. It was decomposed by cold KOH with production of chloroform. At room temperature, it reduced the Fehling liquor. Dichloro antipyrine melted at the same temperature as mono antipyrine and reacted in the same manner with ferric chloride and with KOH (Béhal & Choay, 1892b).
Guaiacol
Béhal and Choay wrote that although guaiacol had become very important due to its therapeutic properties, there was no serious study respect its properties and chemical behavior (Béhal & Choay, 1893a b, 1894b). Analysis of the commercial product sold under that name had shown that it hardly contained 50 of the active components. The rest was composed of a mixture of creosol (the chief constituent of wood-tar creosote) and cresols. For this reason, they decided to prepare it in a pure form, starting from pyrocatechol. A solution of sodium in methanol was mixed with another of pyrocatechol in methanol and then heated in an autoclave to 120o-130 oC with a slight excess of methanol. The resulting product was cooled, distilled to eliminate the alcohol, and then steam distilled to separate the active products. The fraction boiling at 205o- 207 oC, composed of pure guaiacol, was collected and left to crystallize. Guaiacol was a white substance, crystallizing as prisms presenting 12 faces, having a sweet taste, melting at 28.5 oC, boiling at 205.1 oC (760 mmHg), and having relative density 1.143 (0 oC). It was very soluble in anhydrous glycerin and petroleum ether, but little soluble in medical grade glycerin (containing about 3% water). Contact with the tongue resulted in a strong constriction (Béhal & Choay, 1893a b, 1894b).
Creosotes
Béhal wrote that the materials known as creosotes came from two sources, coal and wood. Those originating from wood differed from those coming from coal in that they contained pyrocatechol (Béhal, 1895c). In 1832 Carl von Reichenbach (1788-1869) reported their discovery during the distillation of wood tar; he considered it to be a definite body, boiling at 203 oC and having relative density 1.037 to 1.040 (Reichenbach, 1832). In 1858 Heinrich Hlasiwetz (1825-1875) treated the creosote obtained from beech tree with alcoholic KOH and obtained a crystalline product, which he decomposed into two different substances that he named cresol and guaiacol. The latter was the same product obtained during the distillation of the guaiac resin (Hlasiwetz, 1858). In 1868 Siegfried Marasse (1844-1896) separated by distillation the creosote in different fractions and reacted each of them with zinc in powder; he was then able to identify the presence in creosote of phenol, guaiacol, cresol, cresylol (CH3-O-C6H6-CH3) and a body he named phlorol (Marasse, 1868).
The complexity of the substance creosote led Béhal and Choay to try to develop a separation method based on physical operations such as extraction, steam distillation, distillation, and crystallization. For this purpose, they carried on a series of experiences using pyrocatechol, homopyrocatechol, monophenols, and polyphenols, alone or mixed. From these experiments they obtained the following facts: (1) At room pressure, HBr was able to demethylate completely the methyl ethers of phenols, (2) monophenols were easily entrained by steam distillation while polyphenols were practically not carried over, (3) diethyl ether was able of extracting completely the pyrocatechol, homopyrocatechol, and the monophenols present in an aqueous solution, and (4) benzene was able to separate completely pyrocatechol from homopyrocatechol (Béhal & Choay, 1893b). These facts led them to develop the following analytical procedure: HBr was bubbled through hot creosote in the presence of a certain amount of water. The mixture was then steam distilled, the monophenols passed over while the diphenols remained in the residue. Both liquid phases were then extracted with ether; one of these extractions allowed obtaining the monophenols, the other, the diphenols. The pyrocatechol and homopyrocatechol were then extracted with benzene. This procedure was particular useful for guaiacol (Béhal & Choay, 1893b).
Béhal an Choay went on the develop an analytical method for determining the quantitative analysis of medicinal creosote and the creosote obtained from beech tree and oak tree (Béhal & Choay, 1894d; Béhal, 1895c). Since creosote was composed of a mixture of monophenols and the monomethylic ethers of diphenols, fractional distillation was not a viable process for obtaining highly pure components. The first step was the separation of the monophenols from the monoethers. For this purpose, the creosote was heated in an autoclave with a solution of saturated HCl at 0 oC, for 4 to 5 hours at 180 oC. This resulted in the hydrolysis of the ethers and separation of methyl chloride. The product of this stage was then subject to steam distillation; the monophenols were entrained while the diphenols practically not. The passing liquid was subjected again to the same process. HBr was equally efficient, as proved in the previous publication (Béhal & Choay, 1893b). The monophenols were separated by fractional distillation. The phenol produced melted at 40 oC. The fraction passing at 185o-190 oC was treated with ammonia and other reagents and yielded o-cresol. The fraction passing at 198o-200 oC was transformed into its benzoate, and after purification yielded m-cresyl benzoate. Similar treatments yielded p-cresyl benzoate, o-ethyl phenol, and 1,3,4-m-xylenol and 1,3,5-m-xylenol. Béhal and Choay stopped here (Béhal & Choay, 1894d).
According to Béhal, the monomethyl ethers of diphenols yielded with terreous bases and alkaline-terreous bases (i.e., CaO, SrO, BaO, and MgO) salts that were essentially insoluble in water and methanol, while the corresponding salts of the monophenols were soluble (Béhal, 1895c).10 Béhal and Choay used this fact to carry on their separation. Their results indicated that the creosote from beech tree contained, in round numbers, 40% of monophenols, 25% of guaiacol, and 35% of cresols and homologues, while the corresponding numbers for the creosote from oak tree were 55%, 14%, and 31%, respectively. Hence, the creosote from beech tree had a lower density and a large content of monophenols than the creosote from oak tree. In addition, Béhal and Choay calculated that 100 parts of beech creosote, boiling between 2000 and 210 oC, contained 5.20 parts of ordinary phenol, 10.40 of o-cresol, 11.60 of m- and p-cresol, 3.06 of o-ethylphenol, 2.00 of 1,3,4-m-xyenol, 1.00 of 1,3,5-m-xylenol, 6.20 of other unidentified phenols, 25.00 of guaiacol, and 35.00 of cresol and homologues (Béhal & Choay, 1893c d, 1894c; Béhal, 1895c).
Béhal and Choay published two books summarizing their results on the subject (Béhal 1895cc; Choay & Béhal 1894).
Asbolin (acrid oil obtained from wood soot)
Béhal and Paul Desvignes wrote that soot had long been used in medicine for the treatment of a large number of illnesses (Béhal & Desvignes, 1892). Henri Braconnot (1780-1855) had brewed the soot with a hot solution of HCl and extracted the liquid with ethanol. The alcoholic solution was evaporated to dryness and the residue extracted with ether. Evaporation of the ethereal extract left a clear yellowish syrupy liquid of a nitrogenous substance, extremely acrid and bitter that Braconnot named asbolin (Braconnot, 1826). According to Béhal and Desvignes, it was clear that Braconnot product had to contain acids (simple or complex), phenols, neutral bodies soluble in water, and salts of bases soluble in ether. As a first step, they boiled an alcoholic solution of asbolin with lead carbonate, to eliminate the possible acids present, and then decomposed the lead salt with diluted sulfuric acid. They promptly found that the resulting acid mixture contained an abundant amount of acetic and butyric acid. The alcoholic solution was distilled under vacuum; the first fraction contained most of the alcohol; the two following ones, boiled at about 154o-155 oC and 158o-160 oC (30 mmHg), respectively. These two bodies could be distilled at atmospheric pressure without decomposition; the lighter one boiled at 240 oC and melted at 104 oC. Elemental analysis indicated that it contained, by weight, 65.22% carbon, 5.79% hydrogen, and 28.99% oxygen, corresponding approximately to the formula C6H6O2. It was soluble in water, its alcoholic solution turned dark green with ferric chloride, and its aqueous solution reduced the Fehling liquor. All these results served to identify it as pyrocatechol. The second substance boiled at 251o-252 oC (750 mmHg), crystallized very slowly, melted at 46 oC, and was very soluble in water, alcohol, benzene, and acetic acid. It was insoluble in petroleum ether. Recrystallization from a mixture of petroleum ether and benzene yielded a crystalline matter melting at 51 oC. Elemental analysis indicated that it contained, by weight, 67.32% carbon, 7.64% hydrogen, and 25.04% oxygen, corresponding approximately to the formula C6H8O2. With ferric chloride, chloroform, and KOH it gave the same-colored reactions as pyrocatechol, so that it should be considered to be a homopyrocatechol. Béhal and Desvignes remarked that it was interesting to notice that asbolin, which was still being used as a medicine against tuberculosis, contained the two phenols that as methyl ethers, constituted creosote: The pyrocatechol corresponded to guaiacol and homopyrocatechol to cresol (Béhal & Desvignes, 1892).
Action of organometallic derivatives
Béhal wrote that he had found that the alkyl halogen derivatives of magnesium reacted with ethers of the cyclic series in three stages (Béhal, 1901):
(1) a molecule of the organometallic halogenated derivative became fixed to the ether
R-COOC2H5 + IMgCH3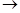 R-C(CH3)(OMgI)(OC2H5)
R-C(CH3)(OMgI)(OC2H5)
(2) a second molecule of the derivative became equally fixed, with elimination of a molecule of the iodoethoxy magnesium
R-C(CH3)(OMgI)(OC2H5) + IMgCH3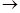 R-C(CH3)(OMgI)-CH3 + IMgOC2H5
R-C(CH3)(OMgI)-CH3 + IMgOC2H5
(3) an ethylenic hydrocarbon (pseudo-propylenic chain) was formed
R-C(CH3)(OMgI)-CH3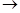 R-C(CH3)=CH2
R-C(CH3)=CH2
These results were not affected by the presence of phenol or phenol ether groups in the nucleus. The resulting bodies polymerized easily generating mostly well-crystallized dimers. These dimers originated during the reaction that produced the monomers; the union of the two molecules occurred through the double bond. These dimers distilled without alteration and heated slowly they split into two molecules of dimers. Permanganate or the chromic mixture oxidized substances having a pseudo- propylene chain yielding methyl ketones:
R-C(CH3)=CH2 + O2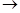 R-CO-CH3 + CH2O2
R-CO-CH3 + CH2O2
Treated with iodine or mercuric oxide, in the presence of alcohol, they transformed into ketones that combined with sodium bisulfite. The pseudo-propylene chain experimented a molecular transposition that changed it into a propylene chain (Béhal, 1901):
Béhal and Marc Tiffenau reacted methyl magnesium iodide with methyl anisate and obtained a monomer melting at 32 oC and boiling at 224 oC, and a dimer melting at 58 oC, which at 350 oC changed slowly into the monomer. Treatment of p-pseudo-propenyl anisole, CH3-O-C6H4-C(CH3)=CH2, with iodine and mercuric oxide, in the presence of alcohol, yielded para-methoxyphenyl ketone, CH3-O-C6H4-CH2-CO-CH3. This ketone boiled at 263o-264 oC, combined with sodium sulfite, and the product was decomposed by water (Béhal & Tiffenau, 1901).
In a following paper, Béhal and Tiffenau showed that carrying carefully the main reaction:
R-COO-C2H5 + 2IMgCH3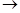 R-C(CH3)2OMgI + IMgOC2H5
R-C(CH3)2OMgI + IMgOC2H5
resulted in the formation of tertiary alcohols, with excellent yields. Some of these alcohols, for example, metamethoxy-2-phenyl 2-propanol and 2-veratryl-2-propanol, were crystalline (Béhal & Tiffenau, 1904).