Introduction
Life and career (Glaeser, 1878; Bilotte, 2014, Paquot-Marchal, 2015)
Jean Pierre Édouard Bernard Filhol (Figure 1) was born on October 7, 1814, in Toulouse (Haute-Garonne, France) and passed away in Toulouse on June 25, 1883. After finishing his basic classic education in Toulouse he studied pharmacy for three years and then moved to Paris where, in 1834, he gained the first place in the competition for an internal pharmacist position in the public hospitals of Paris. During his stay he won the first prize of the internship and of the École Supérieure de Pharmacy. In 1837 he was awarded the first prize in chemistry and botany and the second prize in pharmacy. In 1839 he defended successfully his pharmacy thesis about the action of HCl on a variety of halides (Filhol, 1839). This was ensued by a fast and successful professional and academic career. In 1838 he was appointed chief pharmacist at the Beaujon Hospital and in 1841 professor of chemistry and pharmacy at the École de Médicine and Pharmacy in Toulouse (in spite of his young age), followed by an appointment of professor of chemistry at the Faculty of Sciences of Toulouse, a position he kept until his death 1883 [he was replaced by Paul Sabatier (1854-1941), one of his former students]. During this period he obtained his degree of docteur ès-sciences from the Faculty of Sciences in Paris, after successfully defending two theses (Filhol, 1841, 1844d), and of docteur ès-médicine, from the Faculty of Medicine in Paris, after defending a thesis about arsenic as a poison (Filhol, 1848). In 1858 he was elected Director of the École de Médicine, while keeping his appointment at the Faculty of Sciences.
Filhol served in many scientific and public positions, among them he was a member of the Académie des Sciences, Inscriptions et Belles-Lettres of Toulouse (1843), serving as its secrétaire-archiviste (1853), director 1854), and President (1855-57); of the Société Impériale de Médecine of Toulouse, Chirurgie et Pharmacie de Toulouse (1843), serving as its Vice-president (1854) and President (1856-1865); corresponding member of the Académie de Médecine (1860) and national associate member (1865); member of the Société des Pharmaciens de Toulouse (1849) and its President (1849); member of the Société de Médicine de la Haute-Garonne; of the Conseil d'Hygiène; of the Société d'Histoire de la Pharmacie; founding member of the Société d'Histoire Naturelle de Toulouse (1866); member of the Commission Administrative des Hospices Civils de Toulouse; Head of the École Préparatoire de Médecine et de Pharmacie de Toulouse, founder and curator of the Museum d’Histoire Naturelle de Toulouse (1865-1872), etc. In 1866 he was appointed Chevalier of the Légion d'Honneur and in 1868 promoted to Officier. All these activities did not preventing him of taking an active role in local politics serving as municipal counselor (1860-1866), adjunct mayor (1865-1867), and major of Toulouse (1867-1870). He also served as expert in several forensic cases of toxicology (e.g. Filhol & Couseran, 1851). The work with Nicolas Joly (1812-1885) about milk earned them the 1851-1853 prestigious prize (Gold metal and 600 francs) of the Académie Royale de Médecine de Bruxelles (Joly & Filhol, 1856).
Scientific contribution
Filhol wrote about 60 papers and books (e.g. Filhol, 1853b, 1866, 1877; Filhol & Couseran, 1851) on the subjects of inorganic, organic, and agricultural chemistry, hydrology, vegetable principles, winery, toxicology, paleontology, etc.
In addition to the subjects described below, he studied the preparation of carbon monoxide by the reaction between lactic acid and sulfuric acid (Filhol, 1844b) and of iodoform (Filhol, 1844c, 1845); he developed a method for determining the amount of nitrogen present in flour (Filhol, 1846) and another for detecting the falsification of commercial flour (Filhol, 1847b); he analyzed the relation between atomic mass, crystalline form and density of bodies (Filhol, 1847a); studied the bleaching power of carbon and other substances (Filhol, 1852a, 1855) and the methods for the quantitative determination of hydrogen sulfide (Filhol, 1852b, 1864a); reported the chemical composition of Arbutus unedo (strawberry tree) (Filhol, 1860d); discussed the chemical procedures for determining poisoning by phosphorus (Filhol, 1860e); together with Casimir Célestin Baillet (1820-1900) studied the composition and toxic properties of several species of the gender Lolium (Baillet & Filhol, 1863, 1864); the composition of corn (Filhol, 1867); the double decomposition between soluble and insoluble salts (Filhol, 1873); discussed the different procedures for detecting the alteration or falsification of coffee (Filhol, 1875); etc. Filhol also carried an extensive research on the composition and properties of numerous French mineral water sources (e.g. Filhol, 1853a b, 1866, 1877).
Attention must be paid in what follows to the fact that in all his equations and formulas Filhol assumed the old value of the atomic masses: C = 6, H = 1 and monoatomic, O = 8, N = 14.1, HO = water, etc.
Copal
Filhol wrote that Jöns Jacob Berzelius (1779-1848) and Otto Unverdorben (1806-1873) had studied the properties of copal and not reached the same conclusions (Filhol, 1841, 1842a). According to Berzelius, the copal resin appeared as colorless or slightly yellow pieces, having relative density between 1.045 and 1.139 depending on the source. In was sparingly soluble in anhydrous alcohol but heated with alcohol it swelled and transformed into a viscous elastic substance. It also swelled in ether and then dissolved completely (Berzelius, 1827). According to Unverdorben, copal was completely soluble in alcohol if one part of it was digested for 24 hours with 1.5 parts of alcohol because the resin was soluble in a concentrated alcoholic solution of copal. Fused copal had properties different from the substance in its solid state for it was made to combine with alcohol and turpentine. Unverdorben extracted from the copal of Africa five different resins, none of which was found to have practical use (Unverdorben, 1827).
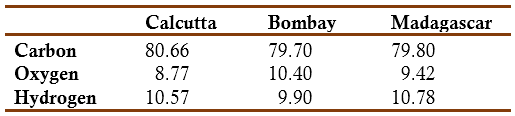
Filhol wrote that the fabrication of copal varnish was a difficult and complicated technique and that it would be of interest to study its chemical composition in order to find a better procedure for dissolving the resin and making a better varnish. He had found that commercial copal appeared in different forms, each having different properties, and that its solubility varied with its age. Distinction was made in commerce between hard and soft copal. The properties of hard copal varied according to its source: (1) the copal from Madagascar appeared in large, flat, lemon-yellow pieces, which were very hard, tasteless and odorless in the cold but diffusing a strong aromatic odor when thrown on incandescent coal; (2) the Indian copal was the one used commonly used; it had a rough surface. The one coming from Calcutta appeared in small flat pieces, nearly colorless, rough and brownish-red on the surface but interiorly quite transparent; (3) some other commercial varieties of copal were thought to come from Brazil and South Africa. Filhol found that the resin coming from Calcutta, Bombay, and Madagascar had the following chemical composition (Filhol, 1841, 1842a):
On the basis of the above results Filhol decided to group the first two species, under the generic name Indian copal. He mentioned that Joseph-Louis Gay-Lussac (1778-1850) and Louis-Jacques Thenard (1777-1857) had reported that copal contained only 76.8% carbon (Gay-Lussac & Thenard, 1810). Filhol believed that this difference was due to the rapid oxidation of the resin when exposed to the atmosphere. He proved this by exposing finely pulverized copal for three days to a stream air at 100 °C. He found that the carbon content had decreased to 76.54%. Another additional result was that finely powdered resin exposed to air for three months became completely soluble in alcohol and ether. Filhol did not test Unverdorben claim that copal, after melting, also became soluble in alcohol. In addition, he found that treating Indian copal with ammonia did not lead to gelatinization, even under the influence of heat (as claimed by Berzelius). However, it did occur immediately upon addition of hot alcohol. Filhol found that concentrated sulfuric acid blackened the copal powder with evolution of heat and release of some SO2. Cold nitric acid in excess dissolved partially the resin; hot acid decomposed it forming a perfect solution (Filhol, 1841, 1842a).
The next experiments were devoted to the separation of the different resins present in copal (Filhol, 1841, 1842a). In the first stage, pulverized copal was extracted with alcohol of relative density 0.895; the solution was filtered and immediately treated with an alcoholic solution of cupric acetate. The resulting green and flocculent precipitate was dried in hot air; at 40o to 50 oC it released a volatile oil and at 100 oC it melted giving off water and volatile oil. Upon cooling it formed a greenish blue transparent friable mass, which was found to contain several resinates. Extracted with ether and evaporation of the blue solution left a residue that Filhol named alpha resin. The portion not precipitated by cupric acetate was found to contain another resin, which Filhol named beta resin. Treatment of the portion of the copal insoluble in alcohol of relative density 0.895 with boiling absolute alcohol resulted in the precipitation of a mixture of alpha and beta resins, accompanied by a third resin that Filhol named gamma resin. The remaining alcoholic solution was first treated with an alcoholic solution of KOH and then with sulfuric acid. This resulted in the precipitation of the gamma resin mixed with an additional resin (delta resin). The remaining final residue was found to contain a fifth resin (epsilon resin), initially insoluble in common solvents, and becoming soluble in time by oxidation. Filhol was unable to decide if the separated alkali resinates were present as such in copal or were a result of the chemical treatment (Filhol, 1841, 1842a).
Filhol then provided a detailed description of the purification and properties of the different resins. Fresh alpha resin was soft and melting at 100 oC into a transparent and lemon yellow substance. On cooling it was very brittle, completely soluble in alcohol of relative density 0.87, in ether, and in turpentine. Elemental analysis indicated that it contained, by weight, 76.61% carbon, 10.20% hydrogen, and 13.19% oxygen, corresponding to the formula C80H62O8. Filhol found that the combinations of alpha resin with base dissolved easily in ether but were insoluble in alcohol, except that of potassium, which was somewhat soluble in water, but from which an excess of alkali precipitated them immediately. Metallic salts produced in the solutions of the alpha-resin gelatinous precipitates of varied colors. The solubility of the ammonium resinate depended on the nature of the salt; it dissolved well in alcohol and lost all its ammonia only after prolonged boiling. This meant that the alpha resin possessed strong electronegative properties (Filhol, 1841, 1842a).
The beta resin was soft, transparent, and melted well below 100 oC. It was lighter than boiling water and dissolved well in alcohol, ether, and turpentine. The softness was probably due to the presence of a large amount of volatile oil, which was possible to eliminate by steam distillation. Elemental analysis indicated that its weight composition was very similar to that of the alpha resin: 76.85-77.04% carbon, 10.08-10.03% hydrogen, and 13.07-12.93% oxygen. Its combinations with bases were very similar to those of alpha resin, except that they were completely soluble in absolute alcohol; they were also soluble in ether (Filhol, 1841, 1842a).
The gamma resin was white, pulverulent; very light, less fusible than the preceding ones and decomposing almost immediately. It was soluble in anhydrous alcohol and ether. Its compounds with bases were insoluble in alcohol or in ether, except for potassium resinate, which was slightly soluble in alcohol and in the alcoholic solution of KOH. The copper copalate was a blue powder and the lead resinate was white and melting with difficulty. Elemental analysis indicated that it contained, by weight, 80.70-80.53% carbon, 10.43-10.66% hydrogen, and 8.87-8.77% oxygen, corresponding to the formula C80H62O3. This was the most abundant resin in copal. The delta resin was obtained in too small quantity for analysis; freshly precipitated it was gelatinous and when dried it formed a white powder, insoluble in alcohol and ether, but soluble in the alcoholic solution of KOH. The epsilon resin appeared as small transparent gelatinous lumps, completely insoluble in ether, alcohol, turpentine, or KOH, and drying into hard granules. It did not combine with bases. Elemental analysis indicated that it contained, by weight, 81.16-81.68% carbon, 10.54-10.43% hydrogen, and 8.30-7.89% oxygen, corresponding to the formula C80H62O2 (Filhol, 1841, 1842a).
Filhol also determined the properties of soft Indian copal, which was sold as white globular drops weighing from 60 to 80 g, externally earthy and impure, but inwardly transparent. It melted at 100 °C, was completely soluble in cold turpentine and sparingly in absolute alcohol. Elemental analysis indicated that it contained, by weight, 85.30-85.36% carbon, 11.50-11.53% hydrogen, and 3.20-3.11% oxygen (Filhol, 1841, 1842a).
Filhol summarized his findings as follows: (1) Exposition of the finely powdered copal resin to air and high temperature resulted in its oxidation, as reported by Unverdorben for African copal; (2) the product of these oxidations were new resins that seemed to derive from the same radical as the primitive resin; (3) the several varieties of copal called Indian copal, had the same composition, with slight differences; (4) Indian copal contained five different resins of which, the oxygenated were the most soluble; (5) the copal resin that was insoluble in alcohol and turpentine became soluble in these solvents after absorbing oxygen; and (6) care had to be taken in the analysis of these resins against the influence of air and water (Filhol, 1841, 1842a).
Capsules of Papaver rheas (poppies)
Filhol wrote that the small species of poppy (Papaver rheas) had been little examined and the presence of morphine in their juice or their extract had not been proved in a clear manner. Alphonse Chevalier (1828-1875) and A. Richard had reported that the poppy flowers contained the alkaloid while H. Riffart had been unable to detect it there (Chevalier and Richard, 1827; Riffart, 1830). Filhol believed that, if at all, the capsule should contain more morphine than the flowers, and this led him to study this possibility (Filhol, 1842b).
Filhol initiated his experiments with capsules collected a little before their complete maturity; unfortunately, the amount of milky juice they yielded by incision was too small. This forced him to examine the entire fruit. The juice of the capsule and of its extract with water was found to be very acid. Filhol also prepared another extract using water slightly acidulated with HCl. Both extracts were examined in the same manner: Fifty grams of extract were dissolved in 150 g of water, followed by heating and addition of an excess of ammonia. The resulting liquor was slightly turbid but did not yield a precipitate. Left by itself for several days it turned into a jelly presenting here and there some brilliant grains. Addition of a small amount of water resulted in the precipitation of the grains, which were insoluble in water and very soluble in diluted acids. Addition of ammonia to the acid solution precipitated the grains as a white powder, insoluble in water and alcohol, and unaffected by heat. The same solution gave a canary yellow precipitate with an ammonia solution of silver nitrate, and a white precipitate with lead salts. The lead precipitate, fused with a blowpipe, presented well-defined crystalline facets; some of these shining grains, fused in the blowpipe with a little of cobalt nitrate, turned blue showing that they contained phosphoric acid and alumina, probably as alumina phosphate. Filhol found that 50 g of extract yielded 0.9 g of grains (Filhol, 1842b).
Additional treatment of the liquors showed the presence of a very small amount of morphine (0.02 g), so that the sedative effect of the capsule extract had to be very small or nil when compared with the same weight of opium. More analyses showed the absence of meconic acid, of an acid (probably gallic acid), and of inorganic salts of potassium, calcium, magnesium, silicon, and iron, joined to the acids sulfuric, HCl, and phosphoric (Filhol, 1842b).
Vegetable and flower pigments
Flower pigments
Filhol published several papers on the nature and properties of vegetable and flower pigments (Filhol, 1854, 1860a b c, 1868a; Filhol & Chatin, 1863; Chatin & Filhol, 1863). In one of them (Filhol, 1860c) he gave a short history of the development of the subject: Robert Boyle (1627-1691) was the first chemist to study the action of acids and alkalis on the coloring matter of flowers and leaves: "We thought fit to make Trial upon the Flowers Jasmin, they being both White as to Colour, and esteem'd to be of a more Oyly nature than other Flowers... Having taken the White parts only...and rubb'd them somewhat hard with my Finger upon a piece of clean Paper...a strong Alkalizate Solution, did immediately turn the almost Colourless Paper Greenish Yellow...(Boyle, 1680). Alexander de Humboldt (1769-1859) observed that these substances formed also in the absence of light and seemed to depend particularly on the amount of oxygen absorbed by the flowers: "Here it follows that the flowers of vegetables with no ray of the sun to be able to dip a variety of colors, it was not the light, but they seem to depend on the oxygen supply..."(Humboldt, 1793). Filhol added that for a long time the botanists had supported the idea that in all vegetables the green color seemed to be the starting point of all others. Thus it could be seen that organs colored green assumed successively the tint yellow, red and orange yellow, or from green to blue, violet, and red (Filhol, 1860c). Augustin Pyramus de Candolle (1778-1841) had proposed classifying vegetable colors in two series, the cyanic and xanthic series. The colors had been ordered in the following two series (Candolle, 1832):

Jean François Macaire Prinsep (1796-1869) had stated that in autumn, the leaves, at the time they lost their green color, stopped developing oxygen; they now absorbed a certain amount of this gas and their tissues generated an acid that colored them first yellow and then red. Macaire Prinsep claimed that saturating the acid with alkali regenerated the green color (Macaire Prinsep, 1826). Ludwig Clamor Marquart (104-1862) stated that all the petals were colored green by chlorophyll as long as they were inside the bud; afterwards the chlorophyll changed and gave birth to new coloring matters that Marquart denominated anthocyanin and anthoxanthin. The first one was present in all blue, red, and violet flowers; it was blue in neutral solutions, red in acid ones, and turned green in contact with acids. Anthocyanin and anthoxanthin transformed one into the other when combining with water or with hydrogen and oxygen in the proportion or water (Marquart, 1835-1836; Filhol, 1860c).
In his first paper Filhol described a series of experiments describing the action of bases and acids on mono-colored flowers (white, rose, and blue) and multicolored ones (Filhol, 1854). Exposing to diluted ammonia the white flowers of Viburnum opulus (Guelder rose), Philadelphus coronaria (mock orange), Chrysanthenum vulgare (common tansy), white roses, and a large number of others, turned them rapidly into vivid yellow and remained as such for a long time. The substance that under the influence of alkalis changed the white color to yellow, seemed to be present in all parts of the flower (e.g. stamen, pistil, etc.), even on leaves deprived of chlorophyll. The best procedure to transform white flowers into yellow ones consisted in placing them in a large vessel full of aqueous ammonia or dipping them in the solution. Placing the petals in a solution of sodium carbonate and a little of cupric sulfate in distilled water generated a yellow gold color, and placing the yellowed flowers in acidulated water reversed the process. Filhol speculated that the responsible substance was probably lutein; it was very soluble in water, a little less in alcohol, and much less in ether (Filhol, 1854).
Red flowers, such as poppies, exposed to boiling water or alcohol, produced a solution tinted violet red, which turned carmine under the influence of acids, even when highly diluted. In contact with ammonia, the acid solution became purple violet, without traces of green. Addition of ammonia to the non-acidulated solution turned it green red. The flowers of Pelargonium zonale turned violet under the influence of ammonia. The red flowers of vervain (Verbena officinalis) produced a violet red solution with alcohol; addition of dry powdered aluminum hydroxide turned the aluminum slightly yellow; acids turned the liquid phase red, and bases blue. These results indicated that the flowers of vervain contained two different substances, which acids turned into yellow and blue, respectively. Filhol also experimented with flowers having a mixture of white and other colors. The alcohol and water extracts of the flowers of Iris, Violet, Peony, Judas tree, etc., curiously were almost colorless, although the petals had been completely discolored. Addition of a small amount of any acid to either of these solutions changed their color to a red much more intense than the color of the original flower. Filhol believed that the discoloration effect was due to a mixture of the juice contained in the colorless cells with that of the colored ones. Boiling water or alcohol destroyed the organization of the flower leading to a mixture of both juices, as shown by the following experiment: He prepared two equal volumes of an alcoholic or water infusion of Peony, slightly acidulated, and diluted one of them in four times its volume of water and the other in four volumes of an infusion of white flowers. The latter turned out to be much less colored than the first one. This meant that the white juices destroyed (or masked) the coloring matter. Filhol believed that the phenomenon was the result of a colorless combination (and not a reduction) of the coloring matter and the elements of the white juice (Filhol, 1854).
In a following publication, Filhol reported that he had used ether to isolate the yellow pigment from a multitude of flowers (Filhol, 1860a c). This treatment precipitated the anthocyanin at the bottom of the flask, as a slightly thick layer colored blue or red, while the xanthogene (mixed with traces of anthocyanin) remained in the upper ethereal liquid phase. It was known that treatment of a lutein solution with acid resulted in notable decrease of its color while treatment with an alkaline solution intensified it. Filhol reported that a solution of the xanthogene behaved in the same manner. He wrote that xanthogene was a pale yellow substance, partly soluble in water, alcohol, and ether. The pertinent solutions were almost colorless; they were yellow only when in high concentration. Alkaline salts colored initially xanthogene yellow which afterwards turned green, under the additional influence of air. Filhol added that lutein also presented all these properties and for these reasons he was convinced that lutein was present in almost all flowers. Nevertheless, for the moment being, he preferred to use the name xanthogene*, until this identity had been proved or disproved.
Filhol looked for xanthogene in perennial trees, etiolated plants (i.e. leaves of chicory, celery, cabbage, etc.), moss, lichens, roots of radish, turnip, carrots, etc. and found only traces or none of it (Filhol, 1860a c).
In another paper, Filhol reported that anthocyanin did not contain nitrogen and seemed to be identical to the œnocyanin that Alexandre Glénard (1818-1894) had extracted from wine (Glénard, 1858; Filhol, 1866). The coloring matter of grapes was the same present in blue flowers and in the external layer of radish. Certain red flowers, like aloes, owed their coloration to a substance different of cyanin; this substance was little soluble in water and ether and very soluble in alcohol. It did not change its color under the influence of bases or acids. Filhol also reported that he had separated a new coloring substance from certain yellow flowers, especially Crocus luteus (Crocus golden yellow). It was an amorphous solid, soluble in water and alcohol, and insoluble in ether (the latter properties distinguished it from xanthine and xanthein), and having a strong dyeing capacity. The superficial layers of the flowers of Anemone pavonina were colored red by cyanin and the deeper ones yellow by xanthine (Filhol, 1860b).
In 1860 Adolphe Chatin (1813-1901) announced the discovery of a new coloring substance (which he named A) that tinted brown the leaves of autumn. Substance A was present in all vegetables, dissolved in an acid juice; it was protected from all possible alteration by most of organic acids and by diluted mineral acids, and turned brown under the influence of alkalis. A microscopic examination of the autumn brown leaves of Æsculus hippocastanum (chestnut tree), Pyrus (pear tree), Juglans regia (English walnut), Tylia (lindens) etc. and those of the grasses, showed that their only difference was that those of the latter contained less browned chlorophyll (Chatin, 1860). In 1863 Chatin and Filhol added the following additional information: (1) Substance A was very avid of oxygen; it existed in al flowers as well as in most organs of the vegetables; (2) a long action of light and air over chlorophyll colored it yellow brown and loosing its ability to return to green under the influence of HCl. This phenomenon took place on chlorophyll still embedded in the vegetable tissue, as well as the one that had been extracted by solvents; (3) whenever it seemed that HCl was able to develop a green color in chlorophyll it was because the latter was mixed with xanthine; (4) the presence of alkalis favored the combined action of light and air on xanthine and was impaired by the action of acids; (5) the surface of young leaves was protected by a wax layer; the amount of this wax decreased during autumn, when the leaves were changing their color; (6) green leaves submerged in ether and then exposed to air, assumed the coloration of a dead leave. This change was faster if the ether was mixed with ammonia. Ammonia like other alkalis, favored oxidation of substance A and the ether removed the superficial wax protective layer; (7) the coloring matter quercitrin was very common. It was present in almost all the herbaceous parts of vegetables and was usually accompanied by tannin and gallic acid, etc. (Filhol & Chatin, 1863).
Filhol also determined the amount of sugar present in the corolla, pistil, and stamen of about 40 flowers, in the state of bud, blooming, and withered. The results appeared in a table reporting the weight % of water and sugar and the state of the flower (Filhol, 1862).
Chlorophyll
Filhol published several papers on the properties and chemical composition of chlorophyll (Filhol, 1864b, 1865, 1868a b, 1874). He mentioned that none of the available procedures allowed him to prepare chlorophyll in a pure state. These were based on the action of HCl concentrated that actually destroyed the substance; the pertinent chemists had not realized this and had actually operated upon the decomposition products (Berzelius, 1837-1838; Mulder, 1844; Morot, 1859). Thus they had reported that chlorophyll was soluble in HCl, while actually it was not; this acid, even in small amounts, totally decomposed chlorophyll. Actually, chlorophyll in the presence of acids went through two consecutive reactions; the first one reported by no one, the second by Edmund Frémy (1814-1894) (Frémy, 1860). Filhol described the two reactions as follows: (1) upon addition of 5 drops of HCl to an alcoholic solution of chlorophyll, the green color disappears and the solution becomes turbid. Filtration separates a solid brown substance from a yellow liquid. Addition of a large amount of acid returns the original green color; if a large amount of HCl is added to the alcoholic solution, the first reaction goes unnoticed; and (2) organic acids such as acetic, tartaric, citric, and oxalic, produce only the first reaction. This is an excellent method for separating the products of the first reaction in a very pure state. If the brown substance and the yellow liquid are separated, both cleaned of acid, dissolved in ether, and mixed again, it does not return the original green liquid. This implies that the result of the action of these acids is more than separating the chlorophyll into two products. It is probably that new substances are formed that were not present in the primitive chlorophyll. According to Filhol, the two successive reactions resulted in the formation of four products: (1) a brown solid insoluble in alcohol; (a) a yellow substance soluble in alcohol; (3) a white substance originating from the action of HCl on the substance isolated from the first reaction; and (4) a yellow substance isolated by means of ether from the liquid produced by the mixture of the yellow substance with an excess of HCl (Filhol, 1864b, 1865).
Filhol wrote that the brown substance was an amorphous solid, insoluble in water and alcohol, even boiling, and soluble in ether. Concentrated HCl turned it green without dissolving it completely. It was rich in nitrogen and was identical with the material that Muller and Morot described as pure chlorophyll. Certain metallic oxides, particularly the one of zinc, in solution with KOH, favored the oxidation of the brown substance and transformed it into a beautiful green material that, unfortunately, was labile under the influence of air and light. The yellow substance was very similar to the xanthine obtained from yellow flowers except that it contained nitrogen. Concentrated HCl transformed it into a yellow and a blue substance that could be separated by filtration. The green parts of plants always contained in a free state, in addition to chlorophyll, the two yellow substances mentioned above. These could be easily separated by means of acids (Filhol, 1865, 1868a).
A more lengthy paper summarized all the above findings and added more detailed information about the action of light, acids, and organic acids upon chlorophyll, as well as about the four substances described above (Filhol, 1868b).
Poisoning by arsenic
In 1844 Filhol read to the Academy of Sciences of Toulouse a paper comparing the different analytical methods recommended for detecting the presence of arsenic in forensic samples, and presenting a new method of his invention (Filhol, 1844a). In the first place he discussed the three conditions that had to be satisfied for a particular analytical procedure in order for the search to be effective and for the different examiners to reach the same conclusion. The first condition, and the most important, was to put the poison under conditions that made it non-volatile; the second was to transform arsenic into its state of maximum solubility; and the third, to separate it from the sample as completely as possible to avoid altering the results and make gross errors. The most stable and less alterable form was obtained by converting the arsenic into its acid or into potassium arsenate. Potassium arsenate was very soluble and, more or less easily separable from the accompanying organic matter. The procedures for separating the poison from the accompanying organic matter could be classified in two large categories: (1) Carbonization methods, where the organic matter was completely destroyed, leaving carbon as the only residue; (2) completely different procedures in which the organic matter was not destroyed but eliminated at a low temperature by its electrochemical precipitation over another metal, or coagulation of the organic matter by means of a liquid that retained the arsenic (Filhol, 1844a).
The best carbonization methods were the ones proposed by Charles Flandin (1803-1887) and Danger (Flandin & Danger, 1840, 1841) and by Mathieu Orfila (1787-1853) (Orfila, 1839). In the Flandin-Danger procedure the organic matter was destroyed by heating it with one-sixth of its weight of pure sulfuric acid and converted into a carbon residue, easy to wash and retaining the arsenic. The residue was then heated with a little of aqua regia to transform the arsenious acid into arsenic acid. Boiling the latter with distilled water separated it from the carbon (Flandin & Danger, 1840, 1841). Unfortunately, this procedure had several disadvantages: (1) part of the arsenic was lost during the process; (2) heating the residue too strongly could lead to a significant loss; and (3) if the matter being examined contained many chlorides, it was very possible that part of the arsenic was entrained during the decomposition with sulfuric acid. It was known that HCl in the nascent state and hot reacted with arsenious acid transforming it into arsenic chloride, which was very volatile. It was important to remember that most parts of the human body contained sodium chloride. It was claimed that these inconveniences could be eliminated by carrying the carbonization in a glass retort connected to a balloon wet with distilled water to retain the small amount of arsenic volatilized. The Orfila method consisted in mixing the sample with twice its weight of potassium nitrate, crushing it to a powder, and adding it in small portions to a crucible held at dark red (about 650 oC) The potassium nitrate provided all the oxygen needed for burning the organic matter and transforming the arsenious acid into potassium arsenate. This procedure lost about the same amount arsenic as the Flandin-Danger one, but the excess of nitrate employed did not guarantee total carbonization (Filhol, 1844a).
The best methods not involving carbonization were those of V. A. Jacquelain and Hugo Reinsch (1809-1884). In the Jacquelain test the organic sample was mixed in a mortar with sand washed with HCl and then diluted in distilled water. Chlorine gas was streamed through the solution to convert arsenious acid into arsenic acid and eliminate the organic matter by coagulation. Afterwards it was filtered, boiled to eliminate the excess of chlorine, and finally tested in the Marsh apparatus (Jacquelain, 1843). Filhol did not believe that chlorine was able to penetrate the organic matter and retrieve all the arsenic present in the fibrous clot. In the Reinsch test the sample was contacted with metallic copper that had been previously acidulated with HCl. As a result, the arsenic precipitated over the metal as a gray power that was then tested in the Marsh apparatus (Reinsch, 1841). According to Filhol, this procedure was vitiated by several defects. First of all, introduction of copper in a liquid assumed poisoned could mislead the expert to believe that it was part of the poisoning process. In addition, the process was not sensitive enough; Reinsch himself had written that it was able to detect only 10-2 parts of arsenic, against 10-6 by other methods (Filhol, 1844a).
All these arguments led Filhol to design his own procedure for detecting arsenic, based on the property of hypochlorous acid to transform instantly arsenious acid into arsenic acid (Filhol, 1844a). The solid organic matter was placed in a porcelain capsule and heated with a solution of KOH to dissolve it completely. The resulting liquid, containing the arsenic as potassium arsenite and the dissolved organic matter, was cooled, diluted in water, and treated with a stream of chlorine that converted immediately the potassium arsenite into potassium arsenate. The chlorine flow was continued until all the organic matter had coagulated as white flakes. It was then filtered and the retained solid washed with water. All the liquids were joined and evaporated to dryness in a water bath. The residue was then tested in the Marsh apparatus. According to Filhol, the resulting spots were substantially purer than those obtained from the matter carbonized with potassium nitrate. In addition, his process did not involve losses of arsenic since it was not carried at high temperatures and potassium arsenate did not form combinations with organic matter (Filhol, 1844a).
The following publication was divided in three sections; the first one described the preparation, composition, and properties of several arsenites (potassium, sodium, barium, calcium, magnesium, iron, lead, and silver) that Filhol had synthesized, the second, the results of several experiments on the absorption of arsenic by vegetables, its distribution among the different organs, and its elimination, and the third, the carbonization process that Filhol had employed for the analysis of arsenic, and a comparison with the methods most used by the experts on the subject (Filhol, 1848). Filhol, for example, prepared two lead arsenites, one by the double decomposition between potassium diarsenite and neutral lead acetate, and the second by the double decomposition between potassium arsenite, AsO3(KO)2 and neutral lead nitrate or acetate. The first salt appeared as a white heavy powder, little soluble in water. Heated to dark red it melted as a slightly yellow glass, perfectly transparent, without release of traces of arsenic or arsenious acid and without production of arsenate. The second arsenite was extremely similar to the first one; they could only be distinguished by chemical analysis (Filhol, 1848).
The experiments on the absorption of arsenic by vegetables showed that arsenic acid was absorbed much faster than arsenious acids. The pertinent chemical analyses indicated that the absorption decreased in the order receptacles > leaves > fruits > stems > petals. Based on an equal weight, mature fruits contained more arsenic than the previous stages. The experiments on elimination of the arsenic were carried on celery-leaved buttercup (Ranunculus sceleratus) because it could vegetate in water for a long time. The results showed clearly that the arsenic was eliminated through the roots (Filhol, 1848). The carbonization of the vegetables was conducted using a mixture of nitric acid and 12 to 15 drops of sulfuric acid for 100 parts of nitric acid. The carbonization could also be conducted with sulfuric acid, as recommended by Flandin and Danger, but required being carried in a closed flask to avoid losses and the problems listed before, particularly the one about the presence of chlorides. The arguments against the Orfila procedure (using KNO3) were also valid with vegetable samples (Filhol, 1848).