INTRODUCCIÓN
Life and career (Chambon, 1934; Jolly, 1969-1977; Anonymous, 2022)
Paul Cazeneuve (Figure 1) was born in Lyon (Rhône) on January 10, 1852, the son of Jean Germaine Jules Cazeneuve, a pharmacist and member of the local court of commerce, and Victoire Adèle Pancera. After finishing his basic education his father gave him his first knowledge of pharmacie and then Paul begun his studies at the École de Pharmacie de Lyon, while preparing for the license ès sciences naturelles, which he received in 1873. The local school did not grant the highest professional level (pharmacien de 1er classe) and to achieve it he had to transfer to the Faculté de Pharmacie in Paris. While in Paris, he won by competition an internship at the Parisian civil hospitals and this allowed him to obtain his diploma of pharmacien de 1er classe in 1875 and the degree of docteur ès-médecine the following year, after defending theses about the extraction alkaloids and about hematin (Cazeneuve, 1875a, 1876). During his studies he was awarded prizes by the Société de Pharmacie de Lyon (1871), the Société de Pharmacie de Paris (1876), the École de Médicine et Pharmacie de Lyon (1873), and the Faculté de Médicine de Paris (1877). In 1876 he was appointed head of chemical works at the chemistry laboratory of the Hôpital de la Charité de Paris, and the following year he transferred to the same position at the Clinique Médicale (Hautes études) of the Faculté Mixte de Médicine et Pharmacie in Lyon, as well earning a position of maître de conférence of organic chemistry (1877-1880). In 1878 he won by competition the position of agrégé (chemical section), the door to an academic career, at the Faculté Mixte with a thesis about the density of vapors from a chemical viewpoint (Cazeneuve, 1878c). His public intervention led to the decision of the Administration des Hôpitaux de Lyon to allow pharmacy interns the task of preparing medicines. He was appointed chief pharmacist to bring this decision into practice. In 1882 he was promoted professor of organic chemistry and in 1893 he was elected to the chair of organic chemistry. At the age of 31, Paul Cazeneuve was elected corresponding member of the Académie de Medicine, and in 1908 promoted to associate member. In 1888 the Académie of Sciences awarded him the Jecker prize for his work in organic chemistry, particularly, about camphor. In 1895 was elected Chevalier of the Légion d’Honneur and in 1921 promoted to Officier.
Among his many public activities he was a member of the Conseil d’Hygiène Departmental of Rhône, President of the Société des Experts Chimistes, and Associé libre of the Société de Pharmacie de Paris. In the late 1800's he took an active part in the French politics of the Third Republic, as radical socialist. In 1894 he was elected conseiller général of the Conseil Général de Rhône and served as its President from 1901 to 1920. He was elected radical deputy of La Guillotière (a district of Lyon) en 1902 and resigned this position after being elected senator of Rhône in 1909. In 1920 he abandoned political life after failing in his attempt to be reelected. In 1904 he was one of the six Vice-Presidents of the executive committee of the Republican Party.
Paul Cazeneuve passed away in Paris, on March 30, 1934.
Scientific contribution
Cazeneuve wrote over 160 papers and books (e.g. Cazeneuve, 1877b, 1886, 1887, 1893, 1896, 1900) on the subjects of biochemistry, organic chemistry, legal medicine, physiology, etc. In addition to the subjects described below, he studied the bark of Hoang-nau (Cazeneuve, 1878a); developed improved procedures for the fast extraction of caffeine (Cazeneuve and Caillol, 1877a), for extracting hippuric acid (Cazeneuve, 1878b, 1879f), for dosing glucose in the blood, (Cazeneuve, 1879c d); and an improved apparatus for the displacement extraction of immediate principles (Cazeneuve and Caillol, 1877b). He studied the toxicology of salicylic acid and of the sulfo of fuchsine and safranines (Cazeneuve, 1879a, Cazeneuve and Lépine, 1885); the influence of phosphorus on urinary excretion (Cazeneuve, 1879g); the lactic fermentation of urine (Cazeneuve, 1880); the sterilization of milk and the lactic fermentation (Cazeneuve, 1895); studied the origin of the coloration and coagulation of milk by heat (Cazeneuve and Haddon, 1895); the total dosage of nitrogen present in urines (Cazeneuve and Hugounenq, 1888b c); the ammonia fermentation of urine and spontaneous generation (Cazeneuve and Lavon, 1877); etc. As customary for candidates to the principal scientific academies, he wrote a booklet describing his research achievements (Cazeneuve, 1879e). Cazeneuve studied in depth the chemistry of camphor and its derivatives, and polyphenol anhydrides; an explosion in one experiment left him blind of one eye.
Alkaloids
The first publication of Cazeneuve on the subject was a comprehensive review of the extraction and detection of a variety of alkaloids (Cazeneuve, 1875a). He wrote that, in general, the extraction of vegetable first principles involved a lengthy series of decoctions or infusions, filtrations, concentrations, followed by treatments with an acid agent and lime, which clearly affected the quality of the product. In 1831 Alexander Guilliermond (1812-1890) had shown that the length of the process could be substantially reduced by treating the wet powdered vegetable directly with lime, followed by evaporation to dryness and extraction of the residue with an appropriate solvent (Guilliermond, 1831). The basic procedure was as follows: A known amount of powdered cinchona bark (say 10 g) was placed in a capsule, humidified with a small amount of hot water, and left alone for a short time to let the water penetrate the lignin. An equal weight of freshly prepared paste of lime was then added to separate completely the quinine from its combinations. This paste also retained, in an insoluble state, most of the foreign materials that could affect the final purity of the alkaloid. The resulting mass was dried and extracted with a solvent. In 1860 Alexander Glénard (1818-1894) and Guilliermond used the above procedure to develop a very accurate analytical procedure for quantifying quinine, using dry ether, free of alcohol, as the solvent. Ether not only was the best solvent of quinine, it acted very fast, it hardly dissolved cinchonine, and did not dissolve the combinations that lime formed with the different coloring substances, tannates, and quinquinas. Glénard and Guilliermond recommended carrying the extraction in a closed vessel to avoid the errors caused by the evaporation of the solvent. The ether extract was then mixed and agitated with a known amount of sulfuric acid of known strength. The quinine passed into the acid phase and transformed into sulfate while an equivalent of amount of acid disappeared. The remaining acid was neutralized with ammonia of known strength. This allowed an easy calculation of the alkali content (Glénard and Guilliermond, 1860). Afterwards, several scientists proposed minor changes to the analytical procedure, based on using different values for the ratio quinine/lime, alcohol, chloroform, etc., instead of ether, etc. (Cazeneuve, 1875a).
Cazeneuve remarked that narcotine and colchicine dissolved easily in ether, emetine was sparingly soluble, and morphine insoluble. It turned out that the solubility of the alkaloid in ether depended of its molecular state. When in an amorphous state, as when obtained when liberated abruptly from the vegetable by means of lime, it dissolved in sufficient amount to be detected in the dry residue of the ether extract. Left to crystallize by spontaneous evaporation of the ether, it left a residue that usually did not re-dissolve in ether. Glénard had shown that quinine heated to 120 oC, so that it became partially fused, dissolved in ether slowly and with difficult. When powdered it agglomerated in ether and hardly dissolved, but powdered in the presence of ether it dissolved rapidly and easily (Cazeneuve, 1875a).
Strychnine
This alkaloid was easily extracted and detected by mixing in a mortar a very small amount of ground nux vomica with a fragment of quicklime and a few drops of water. The resulting limewater reacted promptly with the powdered vegetable and the heat released was enough to dry the mixture spontaneously. The solid was then treated with ether. Addition of an ethereal solution of oxalic acid precipitated the strychnine oxalate, which in contact with a mixture of sulfuric acid with manganese dioxide or potassium dichromate generated the characteristic violet color. Cazeneuve mentioned that this procedure was very appropriate for forensic exams but no so for industrial use, where ether was not a standard material (due to high taxes). This restriction led to the use of alternative solvents, such as coal oils (kerosene) boiling in the ranges 50o to 100 oC and 80o to 120 oC. G. Boiraux and E. Léger showed that these solvents could be used to prepare strychnine, atropine, narcotine, aconitine, veratrine, and cantharidin of high quality (Boiraux and Léger, 1875). In this procedure the ground nux vomica was mixed with half its weight of aerial lime and a little water, and then dried in the stove and extracted with coal oil and diluted sulfuric acid. Heating and then left to crystallize, concentrated the resulting solution of strychnine sulfate. The mother liquor retained the brucine and its mixture with strychnine (igasurin). The latter could be recovered by treating the liquor with alkali (Cazeneuve, 1875a).
Solanine
In 1820 Desfosses discovered this alkaloid in the berries of black nightshade (Solanum nigrum) (Desfosses, 1820). Desfosses prepared it treating its juice a small amount of KOH, NaOH, or ammonia. The resulting grey precipitate, washed with water, dried and treated with alcohol, and evaporated to dryness, left a very pure alkaline matter, slightly pearly, soluble in mineral acids, and precipitated from them by alkalis, in the form of gelatinous flakes. It colored strongly sulfuric acid and thrown over burning coal it burned without leaving a carbonaceous residue. Heated in a tube it melted and swelled considerably while releasing acrid fumes that did not contain ammonia. The new substance turned blue litmus paper reddened by acids; it was soluble in alcohol and combined with acids. All these properties indicated that it should be classified with the organic alkalis; Desfosses suggested naming it solanée (solanine). He also found that it had a strong action on animals: a 6-month old dog, fed with 0.13 g mixed with a small amount of meat, became sleepy and then started vomiting strongly. After about 75 minutes the animal returned to normality (Desfosses, 1820).
Cazeneuve extracted solanine by treating the juice of Solanum nigrum with ammonia; the resulting gray precipitate was filtered, washed and treated with boiling alcohol. The alcohol extract yielded crystalline solanine by evaporation. He also found it in the shootings of potatoes, by treating them for 24 hours with water acidulated with HCl. The resulting liquor was treated with an excess of lime and the resulting precipitate extracted with boiling alcohol. On cooling, the alcoholic extract deposited very pure solanine. He also found that solanine, under the influence of acids, decomposed into solanidine and glucose. Treating a pulp of the shoots directly with lime and digesting the product with boiling alcohol of 93o, yielded the same result. Pure solanine melted between 234o and 235 oC and solanidine at 200 oC (Cazeneuve, 1875a).
The large differences reported for the properties and composition of solanine and solanidine led Cazeneuve and Pierre Breteau (1872-1932) to study this problem. Constantin Zwenger (1814-1884) and A. Kind had reported that the solanine extracted from the shooting of potatoes contained, by weight, 60.21% carbon, 8.28% hydrogen, 29.88% oxygen and 1.63% nitrogen, corresponding to the formula C43H71NO4 (Zwenger and Kind, 1861), while Albert Hilger claimed it contained 59.40% carbon, 10.30% hydrogen, 28.65% oxygen and 1.65% nitrogen, corresponding to the formula C42H87NO15 (Hilger, 1879).
Cazeneuve and Breteau believed that the divergence was caused by use of an erroneous method of preparation (Cazeneuve and Breteau, 1899b). They mixed intimately shoots, not longer than 10 mm long, with half their weight of lime, dried the pulp at room temperature, and extracted the solid with cold alcohol of 93o. The slightly yellow extract was concentrated to a syrup state by distillation under vacuum. Cooling yielded a crop of crystals that was washed with ligroin and ether and then recrystallized three times from boiling alcohol of 95o. The yield was one gram per kilo of shoots. The solanine appeared as silky colorless needles, strongly white, insoluble in water and ether, slightly soluble in cold alcohol and more in warm alcohol, and soluble in acidulated water. It tinted blue litmus paper and melted at 250 oC. Heated to 105 oC it lost 5.52% of its weight, without alteration. Elemental analysis of the anhydrous form indicated that it contained, by weight, 60.30% carbon, 8.67% hydrogen, 28.50% oxygen and 2.53% nitrogen, corresponding to the formula C28H47NO10,2H2O. Hydrolyzed by means of HCl it split into solanidine, melting a 190 oC and soluble in ether, and a sugar compound that yielded an osazone (Cazeneuve and Breteau, 1899c).
Quassin
Quassin is a white bitter neutral principle first isolated in 1835 from the bark of Quassin amara by Ferdinand Ludwig Winkler (1801-1868). Winckler treated an infusion of the bark with calcium hydroxide to precipitate the pectin and other substances and then evaporated the filtrate over a water bath. The residue was extracted with alcohol and the alcoholic extract evaporated by distillation. The yellow solid residue was a bitter crystalline substance, altered by the humidity of the air. It was purified by extraction with a weak solution of absolute alcohol in ether, followed by evaporation of the solvent. The resulting crystalline solid appeared as white opaque prisms, strongly bitter. Cazeneuve treated 100 g of humidified pulverized bark directly with 50 g of lime, which combined immediately with the pectin. The mixture was dried in a stove and the dry residue extracted with ether. This procedure turned out to be inefficient; it was improved by replacing the solvent by alcohol, followed by evaporation and extraction with cold water. Evaporation of the aqueous extract deposited pure quassin (Cazeneuve, 1875a).
Cocaine
Albert Niemann (1834-1861) extracted this alkaloid from the leaves of Erythroxin coca by maceration with alcohol acidulated with sulfuric acid, followed by precipitation with an excess of lime and extraction with ether. Pure cocaine appeared as small colorless prisms, sparingly soluble in water, much more in alcohol, and very soluble in ether. It melted at 98 oC and at higher temperatures it decomposed releasing abundant ammonia vapors. It had a strong alkaline character and applied to the tongue it communicated a temporary insensibility (Niemann, 1860).
Cazeneuve extracted cocaine by treating 100 g of highly pulverized leaves with freshly prepared lime. The mass was then dried and extracted with ether, yielding a slightly green solution caused by the dissolved chlorophyll. The filtrate was evaporated over a water-bath and the residue extracted with water acidulated with HCl. Evaporation led to the precipitation cocaine chlorhydrate, which could be decomposed by calcium carbonate, and the freed cocaine separated with boiling alcohol (Cazeneuve, 1875a).
Veratrine
Wilhelm Meisner (1792-1853), and Pierre-Joseph Pelletier (1788-1842) and Joseph Bienaimé Caventou (1795-1877) discovered this substance simultaneously in the seeds of the Veratrum sabadilla and the roots of the Colchicum Autummale (Meisner, 1819; Pelletier and Caventou, 1820). Cazeneuve extracted it by treating 100 g of sabadilla seeds with 100 g of lime freshly prepared, and remarked that this ratio was necessary to saponify the fatty material contained in the seeds, fast and completely. The resulting paste was dried over a water bath and the residue lixiviated with 500 g of kerosene. The filtrate was treated with water strongly acidulated with sulfuric acid to transfer completely the veratrine to the aqueous phase. Treatment of the latter with ammonia separated the veratrine as a flaky precipitate. This solid was filtrated, washed with distilled water, re-dissolved in alcohol of 93o, and purified further with ether (Cazeneuve, 1875a).
Rhœadine
Oswald Hesse (1835-1917) discovered this alkaloid in the flowers of corn poppy (Papaver rhœas) (Hesse, 1865-1866). Cazeneuve extracted it by treating 500 g of the flowers and stems of corn poppy with 200 g of lime mixed with 100 g of siliceous sand. The resulting green pulp was dried over a water bath and the residue extracted twice with boiling alcohol of 93o. The filtrate, after cooling, deposited a yellow waxy material, insoluble in cold alcohol and soluble in boiling alcohol. The alcohol was mostly distilled and the residue mixed with a small amount of HCl to transform the alkali into its chlorhydrate, while precipitating the remaining waxy matter. The new filtrate was treated with enough aqueous ammonia to saturate the HCl and liberate the alkaloid. This substance was purified by recrystallization from alcohol. The purified rhœadine appeared as white prismatic needles, which were treated with the standard chemicals for eliminating any possible morphine present.
All the results indicated that morphine was not present in any part of the plant itself and not in the petals, while rhœadine was distributed all over the plant. The petals contained only traces of this chemical (Cazeneuve, 1875a).
In 1891 Cazeneuve reported the synthesis of new colored compounds from morphine and codeine (Cazeneuve, 1891a b c). Seven grams of morphine, boiled for several hours with 500 g of methanol or ethanol and 5 g of p-nitrosodimethylaniline, produced a red solution accompanied by the precipitation of crystalline tetramethyldiamidoazobenzene. The filtrate was evaporated to dryness and dissolved in boiling water. The new residue was purified and turned into a violet colored matter, which Cazeneuve believed was comparable to Bindschedler green and originated from the tendency of morphine to give an oxymorphine. This violet morphine was amorphous, sparingly soluble in water, and was precipitated, like most colorants, by concentrated solutions of sodium chloride. It was very soluble in methanol, ethanol, and amyl alcohol. It seemed to be the first dye derived from a natural alkaloid (Cazeneuve, 1891a b),
Codeine violet was prepared by boiling for 300 hours a mixture of 10 g of codeine with 10 g of p-nitrosodimethylaniline chlorhydrate dissolved in one liter of alcohol. Upon cooling, the solution deposited tetramethyldiamidoazobenzene. The filtrate was evaporated to dryness and then boiled with distilled water. The new filtrate was extracted with amyl alcohol, which separated a beautiful violet colorant, while leaving a blue aqueous solution. Evaporation of the amyl extract left the colorant as amorphous plates, little soluble in water and soluble in alcohols and ether. The dye was found to tint directly silk and wool (Cazeneuve, 1891c).
In 1898 Cazeneuve and Barthélemy Moreau reported the synthesis of several aromatic urethanes of coniine, prepared by reaction of 2 moles of the alkaloid with one gram of an organic carbonate. The resulting compounds were non-crystallizable liquids, distilling without decomposition at normal pressure and having the general formula CO(NC8H16)(OR), where R is an aromatic radical, saponifiable by KOH at 150 oC, and decomposable by sulfuric acid, with release of CO2. Cazeneuve and Moreau reported in detail the synthesis of coniine phenyl urethane, coniine guaiacol urethane, and the coniine (- and (-naphthalic urethane (Cazeneuve and Moreau, 1898).
Pterocarpin
All the above experience about principle extraction led Cazeneuve to study the composition of red sandalwood (Pterocarpus santalinus) (Cazeneuve, 1875a). He wrote that this material was usually sold in a powder form, appropriate for its use a tincture. It was very seldom used in pharmacy, and only for coloring plasters. Three publications had reported important results about red sandalwood. Pelletier had separated and described the red dye (Pelletier, 1814), Frédéric Joseph Preisser (1815-1864) had demonstrated that this dye originated from the oxidation of a primitive colorless principle, which he named santaline (Preisser, 1844), and Hugo Weidel (1849-1899) had identified in the wood an immediate principle that he had named sandal, which was odorless and tasteless, insoluble in cold and hot water, carbon disulfide, chloroform, and benzene, sparingly soluble in alcohol, and a little more in ether (Weidel, 1869).
It was known that treatment of an alcoholic tincture of red sandalwood with a dilute solution of limewater resulted in the precipitation of a lac insoluble in alcohol. Separating the solid and washing it with alcohol produced a yellow solution. Spontaneous evaporation left a highly colored residue, partially crystallized, and very soluble in ether, which Cazeneuve decided to study in detail (Cazeneuve, 1875a). He mixed 100 g of wet sandalwood with 50 g of lime, dried the paste over a water bath, and extracted the residue with ether. After drying, the yellow ethereal solution left a crystalline residue impregnated with a yellow resinous matter. A second extraction with more concentrated ether and spontaneous evaporation left a white residue of crystalline long needles. Cazeneuve repeated the procedure with 10 kg of sandalwood and obtained 45 g of crystals. An elemental analysis (in triplicate) indicated that these crystals contained, by weight, 67.85% carbon, 5.52% hydrogen, and 26.63% oxygen, corresponding to the formula C17H16O5. Cazeneuve proposed naming the new substance pterocarpin, in association with Ptercarpus indicus, the generic material that provided it, (Cazeneuve, 1875a).
The next step was a study of the action of different agents on pterocarpin.
Neutral solvents
Pterocarpin was very soluble in ether and was precipitated by alcohol from the solution as an amorphous white snow solid. Pterocarpin was soluble in cold alcohol and more soluble in warm alcohol, from which it precipitated upon cooling, as microscopic crystals. It was sparingly soluble in boiling water and very soluble in ether. Carbon disulfide was the best solvent.
Heat
Pure pterocarpin melted at 83 oC into a colorless liquid; upon cooling it slowly solidified into a very hard solid, hardly scratched by the nails. Heated again, it liquefied only at 120o to 130 oC. At 240 oC it turned brown and began releasing very dense white vapors and distilling light tars and a little of water containing catechol.
Acids
Concentrated sulfuric acid generated a yellow red solution, which afterwards turned purple violet. Cazeneuve considered this reaction to be characteristic of pterocarpin: putting a crystal of pterocarpin in a porcelain capsule and touching it with a rod saturated with sulfuric acid, generated the purple violet tint mentioned above. Exposing the capsule to nitrous vapors produced a strong violet red color; addition of ammonia vapors changed the color instantly to yellow. Water acidified with a few drops of nitric acid had not action on pterocarpin. Treating it with nitric acid of specific gravity 1.2 produced a green color and partial dissolution. Upon boiling, the color changed successively to brown green and brown red. Acetic acid dissolved pterocarpin without entering in combination and coloration (Cazeneuve, 1875a).
Potassium hydroxide
Diluted KOH had no action on pterocarpin. Pterocarpin heated in the presence of potassium hydroxide and potassium permanganate, decomposed completely.
Iodine and bromine
Pterocarpin did not seem to react with iodine, even when dissolved in carbon disulfide. It was attacked by bromine forming a resinous paste and releasing HBr.
Cazeneuve could not reach a conclusion regarding the nature of pterocarpin; the above results indicated that it was not an acid and not a base, and did not seem to be an ester (Cazeneuve, 1875a).
Years afterwards, Cazeneuve and L. Hugounenq studied the pterocarpin in more detail and found that it contained two active principles instead of one (Cazeneuve and Hugounenq, 1887, 1888a, 1889). The extraction procedure was basically the same as before: Powdered sandal wood was mixed with an equal weight of calcium hydroxide, moistened with water, dried on a water bath and extracted with ether. The coloring matter and the resins combined with the lime to form compounds insoluble in ether. The ethereal solution was distilled to dryness and the residue dissolved with the minimum possible of boiling alcohol of 93o. Upon cooling it crystallized into a mixture of two substances, tarnished with resinous matter, which Cazeneuve and Hugounenq named pterocarpin and homopterocarpin (the previous pterocarpin). A second recrystallization yielded the two compounds in an almost pure state, which were purified by extraction with ether and separated by means of cold carbon disulfide, which dissolved only the homopterocarpin. The pterocarpin was found to be soluble in a great excess of boiling carbon disulfide. Cazeneuve and Hugounenq reported that this process yielded about 5 g of homopterocarpin and 1 g of pterocarpin per kilo of sandalwood (Cazeneuve an Hugounenq, 1887, 1889).
Pterocarpin appeared as white lamella crystals, completely insoluble in water and in cold alcohol, but somewhat soluble in the boiling liquid; slightly soluble in ether, from which it crystallized in lamella, insoluble in cold carbon disulfide and slightly soluble in the boiling liquid, and very soluble in chloroform, from which it crystallized in large monoclinic crystals with lævohemihedral faces. A solution of 4.64 g of the substance in 100 g of chloroform had a rotatory power [(]j = -211o. Heated above 145 oC it became pasty and melted at 152 oC, with slight decomposition. Pterocarpin had a neutral reaction, was insoluble in acids and in a concentrated KOH solution and was colored green by nitric acid. Elemental analysis indicated that it contained, by weight, 68.50% carbon, 4.83% hydrogen, and 26.67% oxygen corresponding to the formula C10H8O3 (Cazeneuve and Hugounenq, 1887, 1889).
Homopterocarpin had the same general properties of pterocarpin. It was a white crystalline substance, soluble in ether, chloroform, kerosene, and carbon sulfide, sparingly soluble in cold alcohol and more in the boiling liquid. It was strongly levorotatory; a solution of 4.22 g of the substance in 100 g of chloroform had a rotatory power [(]j = -199o. Heated to 70 oC it became pasty and melted between 82o and 86 oC. It was not attacked by concentrated KOH even at 200 oC; heated with twice its weight of concentrated HCl at 120 oC it yielded a red brown resin. Elemental analysis indicated that it contained, by weight, 70.81% carbon, 5.77% hydrogen, and 23.42% oxygen corresponding to the formula C12H12O3 (Cazeneuve and Hugounenq, 1887, 1889).
The next paper described the action of several agents on homopterocarpin and pterocarpin (Cazeneuve an Hugounenq, 1888a, 1889).
Heat
Upon distillation, both compounds decomposed mostly into the methyl phenols that constitute creosote, and a little into pyrocatechol. The distillate was almost completely soluble in KOH. Addition of HCl separated from the distillate oil having the properties of guaiacol and similar compounds.
Hydrogen chloride
Both compounds were attacked by this acid, cold or hot, and turned first red and then brown. Eventually they become a black solid porous resin, soluble in alkalis.
Hydrogen iodide
This acid, of relative density 2, had the same effects as HCl, yielding the black resin, methyl iodide, and iodine.
Diluted sulfuric acid
At 150 oC, this acid transformed the two compounds into a slightly yellow resin, acid and non-crystallizable, and totally soluble in KOH.
Nitric acid
Cold ordinary nitric acid transformed the compounds into a green resin, slightly soluble in the acid, which seemed to be a nitro derivative of the primitive material. Fuming nitric acid produced a red resin, insoluble in the acid. The two compounds were not attacked by nascent hydrogen, phenylhydrazine, and acetic anhydride (Cazeneuve an Hugounenq, 1888a, 1889).
The above results suggested that homopterocarpin was a condensed polyphenol, probably a polyorcin (Cazeneuve an Hugounenq, 1888a, 1889).
Vegetable principles
Cazeneuve showed that the Guilliermond extraction process was applicable to many other vegetable principles, for example, catechin. Latour and Cazeneuve observed that the astringency of mahogany wood was due to principles identical to those of acacia (Latour and Cazeneuve, 1875). They found that this wood contained catechin and several of its derivatives, more or less well defined. They treated an infusion of the tree with lead sub-acetate until it become colorless, and treated the filtrate with an additional amount of the sub-acetate, which precipitated a perfectly white solid of lead catechinate. This precipitate was separated and decomposed with lead sulfide. The catechin precipitate was washed with distilled water and dried under vacuum. Pure catechin melted at 217 oC and elemental analysis indicated that it contained, by weight, 59.43% carbon, 5.00% hydrogen, and 35.57% oxygen, corresponding to the formula C20H20O9, or C20H18O8.H2O in anhydrous state (Latour and Cazeneuve, 1875).
Cazeneuve extended the research to determine if this catechin did not mask a neutral or basic substance. For this purpose, he treated 100 g of pulverized wood with lime, in order to generate derivatives of the astringent principle, which were insoluble in ether. A crystalline principle, basic or neutral, would always be soluble in ether, no matter how small its amount. Drying the ethereal extract left no residue, indicating the absence of such compounds (Cazeneuve, 1875a).
Caffetannic acid
Cazeneuve and E. Haddon wrote that the exact formula of caffetannic acid seemed to be in doubt. The one usually accepted, C15H10O8, had been proposed by Heinrich Hlasiwetz (1825-1875), assuming that caffetannic acid was a glucoside resulting from the combination of caffeic acid and mannose with loss of one molecule of water: C9H8O4 + C5H12O5 = C15H10O8 + H2O (Cazeneuve and Haddon, 1897; Hlasiwetz, 1867).
Cazeneuve and Haddon studied the reaction of the acid and its sugar with phenylhydrazine and concluded that Hlasiwetz formula was completely wrong and that sugar originating from its split could not be mannose or any other known sugar. Caffetannic acid produced a very pure crystalline osazone that allowed determining its composition. For this purpose, they extracted the acid according to the instructions of Hlasiwetz: A coffee concoction was treated with lead sub-acetate and the resulting yellow precipitate separated, decomposed by means of hydrogen sulfide, and concentrated over a water bath. One hundred parts of the syrupy solution were treated with 12 parts of pure phenylhydrazine and the yellow precipitate separated, washed with alcohol, and dried. The yellow needles, melting at 180 oC, were found to contain, by weight, 62.8% carbon, 5.9% hydrogen, 18.02% oxygen, and 13.28% nitrogen. This analysis agreed very well with the rational formula C6H3(CH=CH-COOH)(OC6H11O5)2 resulting from the union of one molecule of caffeic acid with two molecules of the sugar C6H12O6 and elimination of water (Cazeneuve and Haddon, 1897).
Hematin
According to Cazeneuve, it was known that the normal coloring substance of blood, hemoglobin, was the product of the association of a particular coloring matter named hematin and a little known albuminous substance. It was also accepted that under the influence of most alkalis, even weak ones, diluted acids, and hot water, hemoglobin was modified and became hematin, which remained mechanically connected to the protein principle (Cazeneuve, 1875c, 1876). Scientists, such as Michel Eugène Chevreul (1786-1889), Martial Sanson, and Ger. Joachim Mulder, pretended that hematin did not contain iron and that it was insoluble in water and soluble in alcohol and ether. Others, like Jöns Jacob Berzelius (1779-1848) and Louis René Lecanu (1800-1871), believed that hematin contained iron and was completely insoluble in these solvents. Felix Hoppe Seyler (1825-1895) had confirmed the presence of iron and assigned hematin the formula C96H102N12Fe3O18 (Hoppe-Seyler, 1864). Cazeneuve believed that the divergent opinions were caused by the procedure used to obtain the hematin. Sanson had used concentrated sulfuric acid and pretended to deduce conclusions from the results of this brutal procedure (Sanson, 1835). Today it was known that this treatment led to the formation of compounds similar to the bilirubin of bile. More recently, Claude-André Paquelin (1836-1905) and Léopold Joly had also used a strong procedure (maceration of the dry pulverized globules during eight days with a solution of ammonia in concentrated alcohol) and concluded that hematin did not contain iron. The hematin residue was then digested for several hours with glacial acetic acid, and then inspected for the presence of iron by means of citric acid (Paquelin and Joly, 1874). Cazeneuve repeated the experiments of Paquelin and Joly and concluded that the conditions employed resulted in altered products of hematosine. Boiling of hematin with acids in the presence of water resulted always in a modification of the immediate principle; minerals acid did it more strongly than organic ones. Given the accumulated evidence, Cazeneuve saw no problem in accepting the presence of iron in hematin (Cazeneuve, 1875c, 1876).
Charles Robin (1821-1885) and Verdeil reported a procedure that generated a product soluble in alcohol and in ether (Robin and Verdeil, 1853). Coagulated blood was pressed and then treated with a diluted solution of sodium carbonate in alcohol. The red alcoholic solution was filtered and treated with lime; the latter entrained the coloring matter as a lac that was decomposed with HCl. The albuminous coagulum was washed with ether and then extracted with boiling alcohol. The alcoholic solution of the coloring matter was distilled and the resulting black residue found to be soluble in alcohol and ether. Cazeneuve repeated this experiment; he isolated the matter soluble in alcohol and ether and found that it was a combination of HCl with a derivative of hematin (Cazeneuve, 1875c). After testing several procedures reported in the literature, Cazeneuve concluded that only the one proposed by Hoppe-Seyler allowed preparing pure and unaltered hematin. Its only disadvantage was that it took several weeks. After much testing, Cazeneuve developed the following modified method: One liter of blood was washed twice with a diluted solution of sodium chloride, at no more than 10 oC, to eliminate most of the albuminous matter. The decanted globules were mixed with twice their volume of commercial ether containing 25-30% of alcohol. Cazeneuve mentioned that the alcohol was responsible for coagulation; ether, pure and anhydrous destroyed the corpuscles, without coagulating the albuminous matter. The coagulum was filtered and treated with an ether solution of oxalic acid. The resulting tincture contained the hematin, fatty matter, lecithin, cholesterol, etc. These impurities were removed by treatment with an ethereal solution of ammonia or gaseous ammonia. All these modifications reduced the length of the Hoppe-Seyler process from several weeks to 3 to 4 days (Cazeneuve, 1875c, 1876).
The resulting hematin appeared as a bluish black amorphous powder, odorless and tasteless, insoluble in water, alcohol, pure ether, and acidified water. It was altered by strongly acidified water, alcohol, or ether, and not modified by organic acids. It dissolved in a weak aqueous solution of NaOH, KOH, and ammonia, and formed green lacs with the oxides of lead, zinc, and aluminum (Cazeneuve, 1875a, 1876).
Cazeneuve reported the synthesis and properties of several crystalline derivatives of hematin with HCl, HBr, and HI, including the pertinent halohydrides. He also stated that hematin was a principle containing five elements, carbon, hydrogen, oxygen, nitrogen, and iron (Cazeneuve, 1875c, 1876).
A following publication reported the action of sodium hydrosulfite (sodium dithionite) on hematosine (Cazeneuve, 1877a). Cazeneuve wrote that a spectroscopic analysis of a solution of hematin in distilled water containing ammonia showed the characteristic bands of alkaline solutions of hematosine. Upon addition of a very diluted solution of sodium hydrosulfite, the dichroic tint of the solution disappeared and was replaced by a red vermeil one, which could be confused with the color of a solution of oxyhemoglobin. According to Cazeneuve, this reaction should be very valuable in legal medicine for the characterization of blood. For this purpose, it was enough to treat the suspected blood spot with aqueous ammonia and check in the spectroscope the action of sodium hydrosulfite: Diluted solutions of hematin, hardly showing the band 90 to 100, gave now the neat band of reduced hematin going from 108 to 115 (Cazeneuve, 1877a).
Cazeneuve wrote that it was generally accepted that the biliary and urinary pigments originated from the coloring matter of blood. All illnesses that resulted in the decrease of the blood globules were accompanied by an increase of urobilin (urochrome), the urinary pigment. The vast effusions of blood, present under the skin as a trauma result, showed local transformations of the coloring matter of blood. Many cases of biliary jaundice, which could not be justified by bile resorption, were explained by a transformation of the blood hematin into bilirubin and impregnation of the teguments. This argument had been tested, with contradictory results, by injecting blood subcutaneously to animals. Cazeneuve decided to use this technique to determine the relation of hemoglobin with the biliary and urinary pigments (Cazeneuve, 1876, 1879b). For this purpose, he took two rabbits, subjected to the same food regime, and injected them subcutaneously, one with blood serum and the other with defibrinated blood. Daily analysis of the urines of the second rabbit showed no increase of the urinary pigments and no appearance of biliary pigments. He repeated the experiment injecting this time hematin. Again, the urine analysis did not show the presence of bilirubin and an increase in the amount of urobilin. According to Cazeneuve, the results of his experiments (repeated many times) proved that subcutaneous injections were inappropriate to prove the possible transformation of the blood pigment into urinary or biliary pigments (Cazeneuve, 1876, 1879b).
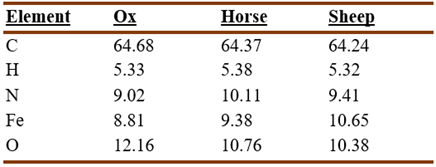
The variety of hemoglobin present in the different animal species led Cazeneuve and Breteau to look into the possibility that there was a similar variation in the composition of their hematin (Cazeneuve and Breteau, 1899a). Analysis of the hematin of ox, horse, and sheep, produced the following results:
The nitrogen and iron content were sufficiently different to allow identifying the animal (Cazeneuve and Breteau, 1899a).