Introduction
Aloe-emodin (3-hydroxymethyl-1,8-dihydroxy-9,10-antraquinone, 1) is a well-known natural compound widely used as an intermediate in the preparation of several therapeutically active compounds such as rhein and diacerein 1, and certain anthracycline type antibiotics.2 Aloe-emodin and derivatives thereof have also been described to be useful in the treatment of cancer 3-9 and psoriasis 10, and as antifungal 11, antiviral 12-14 and antiplasmodial agents.15) Amines are arelatively small group among the reported derivatives of aloe-emodin.5,16-18) They have been described to have antitumor activity.5,16-18) The reported compounds were synthetizedvia substitution reactions from the aloe-emodin bromide or chloride (or the corresponding 1,8-dialkylated derivative) (see figure 1).5,16-18) The experimental yield of this reactions is not high, as it would be expected from substitution reactions, which undergo side-reactions oftentimes.
Another approach to the synthesis of amine derivatives from aloe-emodin is the reductive amination of the aloe-emodincarbaldehyde (figure 1). Although the reductive amination of the 9,10-anthraquinone-2-carbaldehyde using sodium triacetoxyborohydride as reducing agent has been reported in literature 19, the reductive amination of the 1,8-O-protected aloe-emodin carbaldehyde to obtain amino derivatives had not been reported yet. On the other hand, the synthesis of a fewimines from aloe-emodin carbaldehyde can be found in literature 20, but not their reduction.
Reductive amination is a well-known procedure. Several conditions and reducing agents have been reported for this type of reaction. Hydride reducing agents such as sodium triacetoxyborohydride, sodium cyanoborohydride and sodium borohydride have been thoroughly used as reducing agents for their selectivity.21,22 Other reagents have been reported for reductive amination, whichincludeborane-pyridine 22, Ti(OiPr)4/NaBH3CN 23, borohydrideexchange resin 24 and NaBH4/Mg(ClO4)2.25
Herein, the indirect reductive amination of aloe-emodin using different reducing agents and conditions is reported. The synthesis of two new imines derived from 1,8-O-dimethyl aloe-emodin carbaldehyde is reported as well.
Experimental section
Analytical HPLC was performed on a Chromaster HPLC, equipped with a Chromolith® HighResolution RP-18 endcapped column (5 cm x 4.6 mm). Eluting products were detected by a Chromaster HPLC 5430 diode array detector at a wavelength of 214 nm. The mobile phase consisted of 0,1% trifluoroacetic acid (TFA) in acetonitrile and 0,1% TFA in Milli-Q water. Elution through the column was performed using a gradient from 1% to 100% of CH3CN over 5 min at a flow rate of 3 mL/min.
LC/MS samples were analysed on a Waters 2695 Separation Module using a RP C-18 column (Grace Vydac MS, C18, 3 µm, 15 cm x 2.1 mm) at a flow rate of 1 mL/min. Peaks were detected using a Waters 2489 UV Visible Detector (215 nm). Mass spectra were acquired with a MicromassQTof micro time of flight spectrometer, using electrospray ionization (ESI). Data analysis was performed with MassLynx 2.22 software.
TLC was carried out on plastic sheets precoated with silica gel 60F254 (Merck); the spots were visualized under UV light (( = 254 nm).Melting points were acquired on a Buchi Melting Point B-540. IR absorption spectra were recorded on a Thermo Nicolet 6700 FT-IR spectrophotometer.1H NMR and 13C NMR spectra were recorded on a BrukerAvance DRX 250 console and a BrukerAvance II 500 console at 250 or 63 MHz. The deuterated solvent is mentioned in the analysis section and tetramethylsilane was used as an internal standard. In solvents without TMS, the solvent peak was chosen as a reference value. Chemical shifts (() are given in parts per million (ppm), coupling constants (J) are given in Hertz (Hz).
Unless explicitly mentioned, all reagents were purchased and used without further purification and reactions were performed without specific drying of solvents or use of an inert atmosphere.
Synthesis of methyl protected aloe-emodin2
The synthesis of 3-(hydroxymethyl)-1,8-dimethoxyanthracene-9,10-dione 2 was conducted similarly to a literature procedure.17 To a suspension of aloe-emodin1 (1.0 g, 3.7 mmol, 1 equiv.) in acetone (200 mL) was added anhydrous K2CO3 (2.5 g, 18.1 mmol, 5 equiv.) and dimethyl sulphate (1.73 mL, 18.2 mmol, 5 equiv.), and the mixture was refluxed overnight. After 16 hours, more anhydrous K2CO3 (2.5 g, 5 equiv.) and dimethyl sulphate (1.73 mL, 5 equiv.) was added and the mixture was refluxed for additional 6 hours. The reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature and filtered. The filtrate was evaporated in vacuo to afford the crude product as a yellow solid. Recrystallization of the yellow solid from acetone gave compound 6 as yellow needles, yield 75%; m.p. 224-225 °C; 1H NMR (250 MHz, d6-DMSO) δ (ppm): 3,91 (s, 6H, 2OCH3), 4,62 (s, 2H, CH2O), 5,53 (1H, OH), 7,21 (1H, s, H-Ar), 7,30 (1H, d, H-Ar), 7,58 (1H, s, H-Ar), 7,61 (1H, d, H-Ar), 7,74 (1H, dd, H-Ar). The obtained spectrum is consistent with literature data.26
Synthesis of the 4,5-dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carbaldehyde 3
To a mixture of 3-(hydroxymethyl)-1,8-dimethoxyanthracene-9,10-dione 2 (1,1 g, 3,7 mmol) and manganese dioxide (16,09 g, 185 mmol) was added 1000 mL of acetone. The reaction mixture was stirred for 8 hours at room temperature, filtered over Celite, and the solvent was evaporated in vacuo. The product was purified by recrystallization from acetone to afford a yellow solid, yield 62%; m.p. 195-196 °C; 1H NMR (250 MHz, DMSO- d6) δ (ppm): 3.92 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.56 (d, 1H, H-Ar), 7.75 (m, 2H, 2H-Ar), 7.92 (s, 1H, H-Ar), 8.18 (d, 1H, H-Ar), 10.15 (s, 1H, CHO). The obtained spectrum is consistent with literature data.27
General procedure for the synthesis of imines 4 and 5
4,5-dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carbaldehyde 3 (1.0 equiv) and a primary amine (5.0equiv) were dissolved in methanol. Anhydrous magnesium sulphate (5.0 equiv) was added. The resulting reaction mixture was stirred at room temperature in a flask equipped with a calcium chloride trap, and the reaction progress was monitored by TLC. On the completion of the reaction, the solvent was evaporated in vacuo, the solid was extracted with dichloromethane and filtered. After filtration, the solvent was evaporated to afford the product. No further purification was needed.
1,8-Dimethoxy-3-((prop-2-yn-1-ylimino)methyl)anthracene-9,10-dione 4
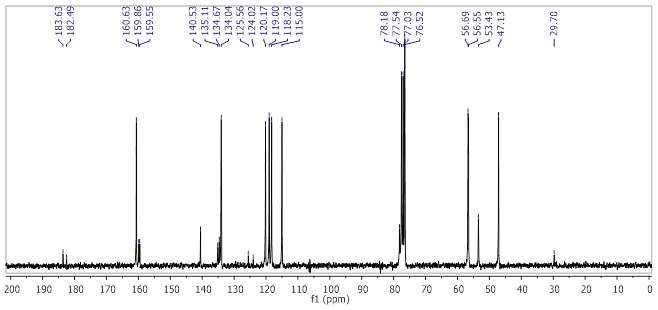
Yellow-orange powder, yield 78%; m.p. 199.7-200.4°C; IR (cm-1, neat): 3248, 3071, 2931, 2874, 2357, 1663, 1580, 1441, 1379, 1333, 1280, 1235, 1158, 1074, 1014, 974, 887, 791, 750, 616; 1H NMR (250 MHz, CDCl3) (see figure 2) δ (ppm): 8.70 (t, J = 1.9 Hz, 1H, CH=N), 8.07 (d, J = 1.3 Hz, 1H, H-Ar), 7.85 (d, J = 6.6 Hz, 2H, H-Ar), 7.65 (t, J = 8.0 Hz, 1H, H-Ar), 7.36 - 7.25 (m, 1H, H-Ar), 4.61 (t, J = 2.2 Hz, 2H, -CH2-), 4.07 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 2.61 (t, 1H, Alkyne); 13C NMR (63 MHz, CDCl3) (see figure 3) δ (ppm): 183.63 (C=O), 182.49 (C=O), 160.63 (CH=N),159.86 (C-OCH3), 159.55 (C-OCH3), 140.53 (Cq), 135.11 (Cq), 134.67 (Cq), 134.04 (aromatic CH), 125.56 (Cq), 124.02 (Cq), 120.17 (aromatic CH), 119.00 (aromatic CH), 118.23 (aromatic CH), 115.00 (aromatic CH), 78.18(C≡C-H), 56.69 (OCH3), 56.55 (OCH3), 47.13 (CH2), 29.70 (C≡C-H).
3-(((3,5-Dimethoxyphenethyl)imino)methyl)-1,8-dimethoxyanthracene-9,10-dione (5)
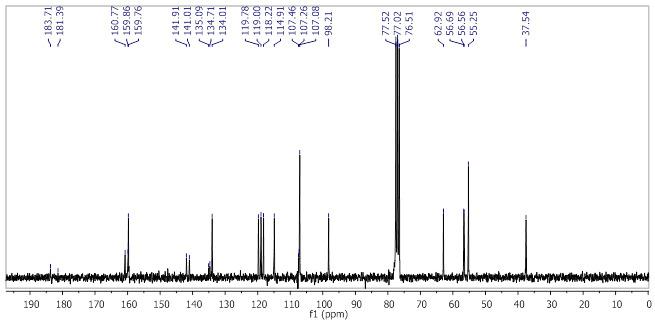
Yellow powder, yield 79%; m.p. 159.5-160.0°C; IR (cm-1, neat): 2959, 2932, 2836, 1668, 1587, 1460, 1319, 1273, 1247, 1203, 1145, 1068, 1027, 969, 800, 745; 1H NMR (250 MHz, CDCl3) (see figure 4) δ (ppm): 8.25 (s, 1H, CH=N), 7.99 (s, 1H, H-Ar), 7.89 - 7.78 (m, 2H, H-Ar), 7.71 - 7.60 (m, 1H, H-Ar), 7.32 (d, J = 8.4 Hz, 1H, H-Ar), 6.40 (d, J = 2.2 Hz, 2H, H-Ar’), 6.32 (t, J = 2.2 Hz, 1H, H-Ar’), 4.07 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 3.92 (t, J = 7.3 Hz, 2H, -CH2-), 3.76 (s, 6H, 2 OCH3), 2.99 (t, J = 7.3 Hz, 2H, -CH2-); 13C NMR (63 MHz, CDCl3) (see figure 5) δ (ppm): 183.71 (C=O), 181.39 (C=O), 160.77 (CH=N),159.86 (C-OCH3), 159.76 (C-OCH3), 141.91 (C-OCH3), 141.01 (C-OCH3), 135.09 (2xCq), 134.71 (2xCq), 134.01 (aromatic CH), 119.78 (aromatic CH), 119.00 (aromatic CH), 118.22 (aromatic CH), 114.91 (aromatic CH), 107.46 (Cq), 107.26 (Cq), 107.08 (2x aromatic CH), 98.21 (aromatic CH), 62.92 (CH2), 56.69(OCH3), 56.56(OCH3), 55.25(2xOCH3), 37.54 (CH2).
Synthesis of amine 6 via reductive amination
Indirect reductive amination
Imine 4 (1.0 equiv.) was dissolved in methanol with 5.0 equivalents of anhydrous magnesium sulphate previously added in a flask equipped with a calcium chloride tub. Then, 10 equivalents of sodium borohydride were added, and the reaction mixture was stirred at room temperature for 30 min. The reaction was quenched using a saturated solution of NaHCO3. Solvent was evaporated under vacuum and the resulting solid was extracted with dichloromethane. The extracts were dried over anhydrous magnesium sulfate and evaporated in vacuum to afford a mixture. The mixture was analysed using HPLC and LC-MS.
In situ stepwise reductive amination
4,5-Dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carbaldehyde (3) (1.0 equiv) and a primary amine (5.0 equiv) were dissolved in methanol. Anhydrous magnesium sulphate (5.0 equiv) was added. The resulting reaction mixture was stirred at room temperature in a flask equipped with a calcium chloride tub. After 2 hours, 10 equivalents of sodium borohydride were added, and the reaction mixture was stirred at room temperature for another 30 min. The reaction was quenched using a saturated solution of NaHCO3. Solvent was evaporated under vacuum and the resulting solid was extracted with dichloromethane. The extracts were dried over anhydrous magnesium sulfate and evaporated in vacuum to afford a mixture. The mixture was analysed using HPLC and LC-MS.
Product: HPLC: RT = 1,85( 0,01 min; LC-MS ([ES+], [C 20 H 17 NO 4 +H] + ): m/z = 336,1225 (calculated m/z: 336,1237)
Results and discussion
Starting from aloe-emodin, two transformations must be conducted before the reductive amination. The first one is the protection of the phenolic hydroxy groups to avoid any influence of the acidity of these groups in the reaction or in the workup after the completion of the reaction. Methyl groups were used as protective group in this case.
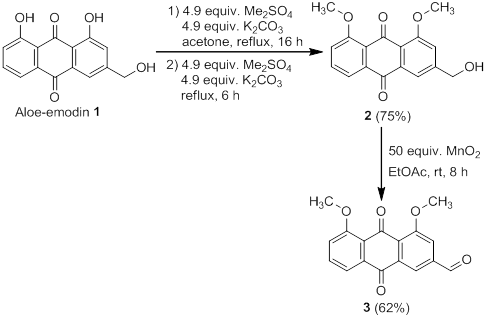
The methylation of aloe-emodin using dimethyl sulfate and a base is a well-known procedure reported in literature.17,26,27 A large excess of dimethyl sulfate and a mild base, to deprotonate only the phenolic hydroxy groups, are used. The low solubility of aloe-emodin in acetone determines the use of large amounts of the solvent. In this work, optimal conditions for the methylation of aloe-emodin included the use of 5 equivalents of Me2SO4and 5 equivalents of K2CO3 (both added in two portions) at 0,04 M concentration of aloe-emodin in acetone. Attempts to increase the yield of the reaction by prolonging the reaction time for longer than 24 hresulted in the appearance of significant amounts of the over-methylated by-product, which is the most common impurity found on the desired product. Recrystallization was used for the purification of the 1,8-O-dimethylated aloe-emodin 2. This purification method was selected due to the increased solubility of the impurities in acetone compared to the low solubility of the desired product. Using these conditions, the product was obtained in 75% yield.
The second transformation that must be conducted before the reductive amination is the oxidation of the 1,8-O-dimethylated aloe-emodin to the carbaldehyde. This oxidation has been reported in literature using different oxidizing agents like pyridiniumchlorochromate (PCC), TEMPO/trichloroisocyanuric acid, VO(acac)2/DABCO/O2 and others.26-29 Yang-Ming et al. reported the oxidation of aloe-emodin 1 to aldehyde in the presence of manganese dioxide in refluxing acetone.30 Aldehyde 3 was synthesized by oxidation of 1,8-O-dimethylated aloe-emodin 2 with an excess of manganese dioxide (50 equivalents) in ethyl acetate (see figure 6) at room temperature, which resulted in the desired aldehyde 3 (gram scale reaction) in good yield (62 %) within 8 h.
Although reductive amination is a well-known reaction in Organic Chemistry, it has not been thoroughly explored for anthraquinoniccarbaldehydes. Only the reductive amination of 9,10-dioxo-9,10-dihydroanthracene-2-carbaldehyde has been reported in literature 19 and there are no reports using polihydroxyanthraquinonescarbaldehydes, including aloe-emodin carbaldehyde 3.
Adbel-Magidet al. published a series of articles describing the several experimental conditions that can be use in this type of reaction.21,22 They reported reductive aminations of aldehyde and ketones in direct or indirect (stepwise) procedures using different reducing agents and gave an insight of the best conditions for obtaining good conversion rates. These results were used in this work.
Since it is believed that reductive amination mechanism (figure 7) includes the formation of an imine intermediate, the first step of the indirect procedure is the formation of the above-mentioned imine. The imine is then reduced in situ or isolated and reduce in different conditions. In this work, both procedures were conducted, along with the direct procedure.
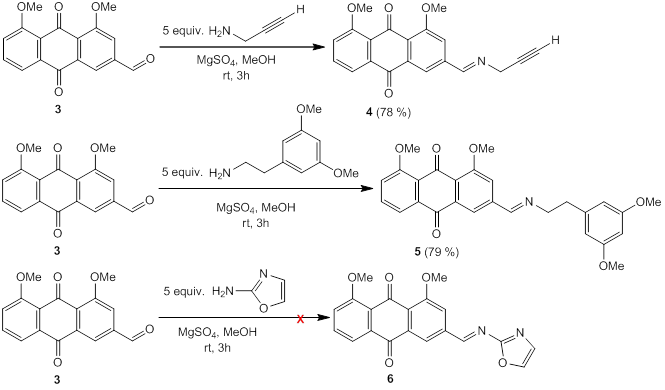
The formation of the imine was tested in methanol. The reaction between aldehyde 3 and propargylamine was conducted in deuterated methanol and followed by 1H NMR. After 1 hour, the signal corresponding to the aldehydic proton disappeared and the signals corresponding to the imine were observed. These conditions were used then to synthesize the imine. Imine 4 was obtained in good yield (78 %) in methanol using magnesium sulfate as a drying agent. Same procedure was tested using another two amines to obtain the corresponding imines. Imine 5 was obtained in a similar yield (79 %) as imine 4, but imine 6 could not be obtained, probably due to the less reactivity of the amine (see figure 8).
Aldehyde 3 was selected for testing the reductive amination considering the protected phenolic groups. Several conditions were tested, starting with the direct reductive amination of aldehyde 3 in dichloromethane using 3 equivalents of sodium triacetoxyborohydrideas reducing agent at room temperature. This procedure was selected following the report in literature of a reductive amination on an anthraquinoniccarbaldehyde.19 After stirring the reaction mixture overnight, no conversion to the amine was observed using HPLC and 1H NMR techniques, only the formation of the imine was observed by 1H NMR. Acid-base catalysts were used then, and the same results were observed (see table 1).
A stronger reducing agent was considered then for the reaction (figure 9). The procedure was conducted stepwise. The aldehyde and the imine were added to the solvent and reacted for 2hbefore adding the reducing agent to the mixture. Using 1.2 equivalents of sodium borohydride in methanol a conversion of 4,1 % was obtained (table 1). An increased in the amount of reducing agent led to an increased in the conversion up to 35-37 %. Same results were obtained using the imine as starting compound.
Table 1 Reductive amination of aldehyde 3 in different conditions
| Reducing Agent | Equiv. | Solvent | Media | Conversiona (%) |
|---|---|---|---|---|
| Na(OAc)3BH | 3 | CH2Cl2 | - | 0 |
| Na(OAc)3BH | 3 | CH2Cl2 | Et3N | 0 |
| Na(OAc)3BH | 3 | CH2Cl2 | HOAc | 0 |
| NaBH4 | 1.2 | MeOH | - | 4.1 |
| NaBH4 | 5 | MeOH | - | 19.9 |
| NaBH4 | 10 | MeOH | - | 34.9 |
| a Conversion refers to area percent for the peak of the amine obtained from HPLC followed by LC-MS of the crude product. |
The reaction (figure 10) was conducted in Argon atmosphere using the same conditions (table 2), considering that the oxygen in the air could influence the formation of the imine from the desired product. The conversion was 20.3 %, which shows that oxygen might not be the reason for the low conversions.
Table 2 Reductive amination of aldehyde 3 in different atmospheres and reaction times.
| Time (min) | Atmosphere | Conversiona (%) |
|---|---|---|
| 60 | Air | 37,4 |
| 60 | Argon | 20,3 |
| 30 | Air | 58,9 |
| a Conversion refers to area percent for the peak of the amine obtained from HPLC followed by LC-MS of the crude product. |
Considering the previous results, the reaction was then conducted in the same conditions, but the reaction time was set at 30 minutes. An increased in the conversion up to 58.9 % was observed. This result suggests the idea that the imine is a thermodynamically more stable product, probably due to resonance stabilization, and it is formed by oxidation of the desired product at longer reaction times.
Conclusions
The reductive amination of 4,5-dimethoxy-9,10-dioxo-9,10-dihydroanthracene-2-carbaldehyde was performed at different conditions. The best conversions (59 %) were obtained using a stepwise procedure, 2 hours for the imine formation and 30 minutes after the addition of the reducing agent, with 10 equivalents of sodiumborohydridein methanol in an open flask. The reduction was also tested starting from the corresponding imine with the same results. Two new imines were synthesised for that purpose