Introduction
Celastraceae family, a group consisting of about 1300 species divided in 106 genera, is widely distributed through warm temperature regions of the world.1,2Maytenus genus is the largest one with around 255 species, 7 it comprises about 200 American species of which 11 grow in Argentina, 77 in Brazil and 10 in Cuba.3
M. elaeodendroides is an endemic plant from Cuba, also known as “Sangre de Toro” (translated as “blood of bull”) and few studies on its biological activity or chemical composition have been conducted. Species of Maytenus genus have a long history in traditional medicine.4 These plants are commonly used as anti-inflammatory (5, analgesic, antiulcerogenic and in the treatment for rheumatism, influenza, gastrointestinal diseases, and skin cancer.6
Triterpenoids from the Celastraceae belonged to the lupane, oleanane, friedelane, taraxerane, glutinane, ursane, dammarane, and baccharane series.7 Lupane triterpenoids are pentacyclic compounds with 30 carbon atoms, biosynthetically derived from the cyclization of squalene, and a vast class of natural products whose structural diversity includes a wide array of functional groups.8
Over the last 30 years or so, phytochemical studies carried out with plants belonging to the Maytenus genus have demonstrated the presence of many classes of constituents, including flavonoids (8-10, pentacyciclic triterpenes principally from the friedelane, oleanane, lupane and ursane series (11,12, sesquiterpenes, alkaloids 13, and condensed tannins.14
Several factors in our body cause the expression of nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) with the consequent generation of large amounts of NO and prostaglandins (PGs) respectively. NO and prostaglandins are mediators of inflammatory processes. The high production of these mediators contributes to tissue damage, leading to chronic inflammatory diseases. It has been described that anti-inflammatory activity in Maytenus genus is associated to the presence of terpenoid compounds. Reyes et al. reported anti-inflammatory properties for triterpenes from several series (including lupane, friedelane, etc. - series) which were isolated from the bark of 2 species of Maytenus.15 By the other hand Spengler et al. isolated triterpenes as friedelane and betuline (lupane-liked) from the bark of the Cuban species M. elaeodendroide.16
Thus we intend to isolate and characterize triterpenes having potential anti-inflammatory activity from the bark of Maytenus elaeodendroides Griseb.
Materials and methods
General
Analytical-grade petroleum ether, diethyl ether, n-hexane, ethyl acetate and dichloromethane were used in this work. Silica gel 0, 06-0,2 mm (70-230 Mesh, Merck) were used for column chromatography and TLC was performed on 0,2 mm-thick Kiesegel 60 F254 layers (Merck). IR spectra were recorded in a WQF-SLO FT-IR (Rayleigh) spectrophotometer in KBr pellets. 1D 1H, 13C, 2D gCOSY, gHSQC, y gHMBC NMR spectroscopy were performed in a Varian Inova spectrometer to 300 y 400 MHz. The spectra were obtained using chloroform (CDCl3) as solvent.
Nature of carbon atoms was assessed by DEPT spectrum edition technique with proton pulse at 1350. Chemical shifts (δ) were expressed in ppm while coupling constants (J) in Hz. δ values were referred to tetramethylsilane as internal reference.
Plant material
The bark of M. elaeodendroides. was recollected in the zone of “La Coca” dam, Campo Florido, Havana, Cuba, and identified by botanic Dr. Pedro Herrera Oliver from the Ecology and Systematic Institute (Havana, Cuba), where a voucher was deposited at the Herbarium under number HAC41417.
Plant extraction and isolation of compounds
The bark of M. elaeodendroides was dried under air circulation (40 0C). Dried-bark powder (950 g) was macerated with petroleum ether/diethyl ether (1:1) at room temperature for nine days. The product of maceration was filtered and concentrated under reduced pressure, yielding 10 g (1,05 %) of the crude of petroleum ether/diethyl ether (1:1). This crude was chromatographed on a silica gel column, using increasing-polarity mixtures of n-hexane/ethyl acetate as eluent to obtain 5 fractions. Fractions F1 and F2 were rich in 3β-friedelinol and betulin, respectively. [15]Fraction F3 was subjected to preparative HPTLC developed with n-hexane/CH2Cl2/Et2O (2.5:1.5:1.5) resulting in compounds 1 (40 mg) and 2 (32 mg). Compounds 3 (35 mg) and 4 (57 mg) were purified by flash chromatography on silica gel of the fractions F4 (petroleum ether/CH2Cl2/Et2O, 3.0:3.0:1.0) and F5 (n-hexane/Et2O, 6.0:4.0), respectively.
Preparing samples
The extract obtained was screened for their topical anti-inflammatory activity at doses of 200 mg/kg; 400 mg/kg and 800 mg/kg. It was used to the compounds 2 and 3 doses of 1, 5 y 10 mg/kg. Croton oil and products under test as well as diclofenac (reference drug) were dissolved in acetone. Diclofenac was used at a dose of 20 mg/kg (≈ 0,5 mg/right ear).
Anti-inflammatory activity assay
Swiss mice (25-30 g), provided by CENPALAB (National Center for Laboratory Animal Production, Cuba), were kept for a week, before the experiment, at constant conditions of temperature (21±1 0C) and humidity (60-70 %), and in light/dark cycle. Water and food were supplied ad libitum, before the experiment. Each experiment group consisted of five animals.
Edema was only induced on the right ear of each mouse. Croton oil (0.125μg/μL) was applied topically to both inner (10 μL) and outer (10 μL) surface of the right ear (total volume administration of 20 μL) simultaneously with the products under study and diclofenac.
Four hours after administration mice were sacrificed by cervical rupture. One circular portion (6 mm diameter) of each ear was cut and weighted in analytical balance. Since in all cases left ear only received vehicle (acetone, 20 μL), subtracting left ear portion weight from right ear portion weight indicated the extent of edema in each mouse. The extent of inflammation was measured as edema weight through arithmetic mean and standard error calculated for each animal group.
Anti-inflammatory activity was assessed by percentage inhibition of inflammation with regard to negative control group. A 100 value was assigned to the group receiving only Croton oil, and the reduction attributed to each of the treated groups was expressed as %. The data was accompanied by the standard error.
Results and discussion
Anti-inflammatory activity of petroleum ether/diethyl ether (1:1) extract
Table 1 reports the edema weight average and standard desviation for each experimental group. Inflammation seen in the negative control group was significantly higher than that seen for the different doses of crude (p< 0,05). The highest dose (800 mg/kg) showed higher inflammation values and had statistical difference with regard to doses of 200 and 400 mg/kg.
Table 1 Extent of inflammation for different doses of petroleum ether/diethyl ether (1:1) extract in Croton oil- induced ear edema in mice
| Groups | Doses (mg/kg) | Extent of inflammation [edema weight average (mg) ± SD] |
|---|---|---|
| Control ( |
- | 118,4±11,78 |
|
|
20 | 14,8±2,17a* |
|
|
200 | 19,6±1,95a* |
|
|
400 | 17,0±7,21a* |
|
|
800 | 38,0±5,83b* |
Different letters represent significant differences (p < 0,05)
*Significant difference compared to control (p < 0,05)
Figure 1 shows the results of inflammation inhibition % by three doses of petroleum ether/diethyl ether (1:1) extract tested.
No significant differences were obtained among the inhibition exhibited by diclofenac (87,5%) and doses of 200 (83,4%) and 400 mg/kg (85,6%). However, there were significant differences (p <0,05) between these groups (diclofenac and doses of 200 and 400 mg/kg) and the one which received a dose of 800 mg/ kg.
It is not clear why the dose of 800 mg/kg of the crude exhibited a lower anti-inflammatory activity compared to doses of 200 and 400 mg/kg despite that a dose of 800 mg/kg still showed an anti-inflammatory response.
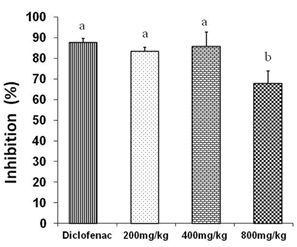
Fig. 1 Anti-inflammatory effects of three different doses of petroleum ether/diethyl ether (1:1) extract in Croton oil- induced ear edema in mice. Different letters represent significant differences (p < 0,05)
Spengler et al., 2002, reported the isolation of triterpene betulin 16, which anti-inflammatory activity was reported in the literature by Reyes et al., 2006 using the method of production of nitric oxide and prostaglandins (PGE2) by stimulation of macrophages.17
Isolation and identification of potentially bioactive compounds
The fractions F1 and F2 rich in 3β-friedelinol, cafeate of betulin and betulin were studied by Spengler et al., 2002.16 Compounds 1 and 2 were isolated from the fraction F3, whereas compounds 3 and 4 were isolated from the fractions F4 and F5, respectively.
The structures of compound 1-4 were elucidated by IR, mono and bi-dimensional NMR spectroscopy, as in earlier reports. (15,16
1:IR (KBr) (ν, cm-1): 3443; 2939; 2871; 1715; 1631; 1464; 1381; 1044; 844.1 H NMR (d-CHCl3), 500 MHz: 4,61(1H,d, J=2,04 Hz, H-29b), 4,51 (1H,m, H-29a), 3,72 (1H,dd, J=10,75 Hz, H-28b),3,27 (1H,d, J=10,75 Hz, H-28a), 2,49 (1H, m, H-2 b), 2,40 (1H, m, H-2 a), 1,90 (1H, m, H-1 b),1,70 (1H, brd, J= 15,0 Hz, H-12 b), 1,64 (1H, m, H-13), 1,61 (3H, s, H-30),1,57 (1H, t, J= 12,5 Hz, H-15 b), 1,48 (2H, m, H-6), 1,43 (2H, m, H-7), 1,42 (1H, m, H-11 b), 1,38 (1H, m, H-1 a), 1,36 (1H, m, H-9), 1,30 (1H, m, H-5), 1,30 (1H, m, H-15 a),1,26 (1H, m, H-11 a), 1,04 (1H, m, H-12 a),1,02 (3H, s, H-23), 0,99 (3H, s, H-26), 0,95 (3H, s, H-24),0,91 (3H, s, H-27), 0,85 (3H, s, H-25).13C NMR (d-CHCl3), 500 MHz: 218,0 (C-3),150,0 (C-20), 109,7 (C-29), 60,5 (C-28), 54,8 (C-5), 49,7 (C-9), 48,6(C-18), 47,7 (C-17), 47,3 (C-4, C-19), 42,8 (C-14), 40,9 (C-8), 39,8 (C-1), 37,4 (C-13), 36,8 (C-10), 34,0 (C-2), 33,9 (C-22), 33,5 (C-7), 29,7 (C-21), 29,1 (C-16), 27,0 (C-15), 26,6 (C-23), 25,2 (C-12), 21,3 (C-11), 21,0 (C-24), 19,6 (), 19,0, 15,9 (C-26), 15,8 (C-25), 14,7 (C-27).
2:IR (KBr) (ν, cm-1): 3354; 2939; 2871; 1705; 1641; 1458; 1383; 1021; 884.1 H NMR (d-CHCl3), 500 MHz: 4,68 (1H, brs, H-29 b), 4,58 (1H, brs, H-29 a), 3,60 (1H, dd, J= 11,2, 4,4 Hz, H-16), 2,50 (1H, td, J=11,4, 5,4 Hz H-19), 2,49 (1H, ddd, J= 16,0, 9,5, 7,5 Hz, H-2 b), 2,40 (1H, ddd, J= 16,0, 8,5, 4,5 Hz, H-2 a), 1,98 (1H, m, H-21 b), 1,90 (1H, ddd, J= 13,0, 7,5, 4,5 Hz, H-1 b), 1,70 (1H, brd, J= 15.0 Hz, H-12 b), 1,68 (3H, brs, H-30), 1,65 (1H, m, H-22 b), 1,64 (1H, m, H-13), 1,57 (1H, t, J= 12,5 Hz, H-15 b), 1,48 (2H, m, H-6), 1,43 (2H, m, H-7), 1,42 (1H, m, H-11 b), 1,40 (1H, m, H-18), 1,40 (1H, m, H-21 a), 1,38 (1H, m, H-1 a), 1,36 (1H, m, H-9), 1,30 (1H, m, H-5), 1,30 (1H, m, H-15 a), 1,28 (1H, m, H-22 a); 1,26 (1H, m, H-11 b), 1,06 (6H, s, H-23 y H-26), 1,04 (1H, m, H-12 a), 1,02 (3H, s, H-24), 1,01 (3H, s, H-27), 0,93 (3H, s, H-25), 0,81 (3H, s, H-28). 13C NMR (d-CHCl3), 500 MHz: 218,0 (C-3), 149,9 (C-20), 109,8 (C-29), 76,9 (C-16), 54,9 (C-5), 49,3 (C-9), 48,6 (C-17), 47,6 (C-18), 47,5 (C-19), 47,3 (C-4), 44,1 (C-14), 40,8 (C-8), 39,6 (C-1), 37,7 (C-22), 37,4 (C-13), 36,8 (C-10), 36,8 (C-15), 34,1 (C-2), 33,5 (C-7), 29,8 (C-21), 26,6 (C-23), 24,8 (C-12), 21,4 (C-11), 21,0 (C-24), 19,6 (C-6), 19,3 (C-30), 16,1 (C-27), 16,0 (C-25), 15,8 (C-26), 11,7 (C-28).
3:IR (KBr) (ν, cm-1): 3400; 2940; 2870; 1690; 1638; 1458; 1383; 1021; 873.1 H NMR (d-CHCl3), 500 MHz: 4.64 (1H, m, H-29b), 4,53 (1H, dd, J= 2,29, 1,44 Hz, H-29a), 3.53 (1H, dd, J= 11,1, 4,9 Hz, H-16), 3,16 (1H, dd, J= 11,5, 4,9 Hz, H-3), 2,49 (1H, m, H-19), 1,67 (1H, m, H-1b), 1,62 (3H, brs, H-30), 1,60 (1H, m, H-2b), 1,56 (1H, m, H-2a), 1,51 (1H, m, H-6b), 1,41 (1H, m, H-11b), 1,39 (1H, m, H-6a), 1,39 (2H, brs, H-7), 1,27 (1H, m, H-9), 1,23 (1H, m, H-11a), 1,19 (2H, m, H-22), 0,98 (3H, s, H-26 ), 0,93 (3H, s, H-27 ),0,91 (3H, s, H-23 ), 0,90 (1H, m, H-1a), 0,76 (3H, s, H-25 ), 0,73 (3H, s, H-24 ), 0,70 (3H, s, H-28), 0,60 (1H, d, J= 10,69 Hz, H-5). 13C NMR(d- CHCl3), 500 MHz: 150 (C-20), 109,6 (C-29), 78,8 (C-3), 76,9 (C-16), 55,6 (C-5), 49,4 (C-9), 48,6 (C-17), 47,6 (C-18), 47,4 (C-19), 44,1 (C-14), 40,8 (C-8), 38,8 (C-4), 38,7 (C-1), 37,7 (C-22), 37,4 (C-13), 36,8 (C-10), 36,8 (C-15), 34,1 (C-7), 29,8 (C-21), 28,0 (C-23), 27,3 (C -2), 24,8 (C-12), 21,4 (C-11), 19,0 (C-30), 18,2 (C-6), 16,0 (C-25, C-26, C-27), 15,0 (C-24), 11,6 (C-28).
4:IR (KBr) (ν, cm-1): 3400; 2940; 2870; 1686; 1638; 1458; 1383; 1021; 873.1H NMR (d-CHCl3), 500 MHz: 4,74 (1H, brs, H-29b), 4,62 (1H, brs, H-29a), 2,50 (1H, td, J=11,4, 5,4 Hz H-19), 2,49 (1H, ddd, J= 16,0, 9,5,7,5Hz, H-2 b), 2,40 (1H, ddd, J=16,0, 8,5, 4,5 Hz, H-2a), 1,98 (1H, m, H-21b), 1,90 (1H, ddd, J= 13,0, 7,5, 4,5 Hz, H-1b), 1,70 (1H, brd, J= 15,0 Hz, H-12b), 1.70 (3H, brs,H-30), 1.64 (1H, m, H-13), 1.48 (2H, m, H-6), 1.43 (2H, m, H-7), 1.42(1H,m, H-11b), 1,38 (1H, m, H-1a), 1,36 (1H, m, H-9), 1,30 (1H, m, H-5),1,26 (1H, m, H-11a), 1,07 (3H, s, H-23), 1,04 (1H, m, H-12a), 1,02(3H, s, H-24), 0,99 (3H, s, H-26), 0,98 (3H, s, H-27), 0,93 (3H, s,H-25).13C NMR (d-CHCl3), 500 MHz: 218,3 (C-3), 181,2 (C-28), 150,5 (C-20), 109,9(C-29), 56,5(C-17), 55,1 (C-5), 50,0 (C-9), 49,3 (C-18),47,5 (C-19),47,0 (C-4),42,6 (C-14),40,8 (C-8),39,8 (C-1),38,6 (C-13), 37,2 (C-7), 37,1(C-10), 34,3 (C-2), 33,8 (C-22),32,2 (C-16),30,7 (C-21),29,8 (C-15),26,8(C-23),25,7 (C-12),21,5 (C-11),21,2 (C-24),19,8 (C-6),19,5 (C-30),16,1(C-25),16,0 (C-26),14,8 (C-27).
Compounds 1 to 4 respectively correspond to: 1 (28-hydroxy-3-oxo-20(29)-lupene), 2 (16-hydroxy-3-oxo-20(29)-lupene), 3 (3ihydroxy-20(29)-lupene) and 4 (acid 3-oxo-20(29)-lupene-28-oic) (figure 2).
Compound 1 (28-hydroxy-3-oxo-20(29)-lupene) was previously isolated from other species of Maytenus genus (14, and it is now isolated for the first time from M. elaeodendroides.
Compound 2 (16-hydroxy-3-oxo-20(29)-lupene) was firstly isolated from Florensia resinosa S.F Blake plant and, later on, from Acacia cedilloi (L) Rico by Pech et al., 2002.18 It is interesting to point out that this is not only the first report of the isolation of this compound from M. elaeodendroides but also from the Celastraceae family.
Compound 3 (3dihydroxy-20(29)-lupene) exhibits interesting medicinal properties such as anti-inflammatory, anti-edematous, antitumoral and anti-HIV activities.19-22 The topical anti-inflammatory activity against the Croton oil-induced ear edema in mice has been reported for this compound, ID50 = 0,2.23 This compound was isolated, for the first time, from M. elaeodendroides.
Compound 4 (acid 3-oxo-20(29)-lupene-28-oic) was isolated from Corma and from other plants belonging to different families, including Celastraceae. The compound has shown different kinds of biological activity, including: cytotoxic activity against a wide range of tumor cells, and antibacterial, anti-malarial and anti-inflammatory activities.24 This compound was isolated, for the first time, from M. elaeodendroides.
Anti-inflammatory activity of compounds 2 and 3
As no report on biological activity had been found for compound 2; while it has been reported anti-inflammatory activity for compounds 1, 3, and 4, we decided to perform an assay for anti-inflammatory activity of compounds 2 and 3 because their structures differs only in the substituent on C-3.
Table 2 shows the extent of inflammation in terms of edema weight average and standard deviation for pure compounds 2 and 3, evaluated independently for their topical anti-inflammatory activity in the Croton oil-induced ear edema model in mice.
Table 2 Extent of inflammation for compounds 2 and 3 in Croton oil- induced ear edema in mice.
| Groups | Doses (mg/kg) | Extent of inflammation [edema weight average (mg) ± SD] |
|---|---|---|
| Control ( |
- | 118,4±11,78 |
|
|
20 | 14,8±2,17a* |
|
|
1 | 46,8±10,76d* |
|
|
5 | 29,8±8,26e* |
|
|
10 | 30,4±1,82e* |
|
|
1 | 115,2±7,12b |
|
|
5 | 98,2 ±6,98 c* |
|
|
10 | 40,6±5,94d* |
Different letters represent significant differences (p < 0,05).
*Significant difference compared to control (p < 0,05)
Table 2 shows that both compounds 2 and 3 significantly decrease inflammation. All doses of compound 2 showed anti-inflammatory activity compared to control group (Croton oil), whereas only the lowest dose of compound 3 (1 mg/kg) did not show a significant decrease in inflammation.
Diclofenac showed the strongest anti-inflammatory activity compared to the doses evaluated for compounds 2 and 3.
Figure 3 shows the results of percentage of inflammation inhibition at different doses of compounds 2 and 3. It was seen an anti-inflammatory activity for both triterpenes. However, diclofenac showed 87,5 % of inflammation inhibition, differing significantly from doses of 1,0, 5,0, 10,0 mg/kg for both compounds. Between the studied triterpenoid compounds best results were obtained for doses of 5,0 (74,83,%) and 10,0 mg/kg (74,3 %) of compound 2, so this compound has a marked anti-inflammatory effect. Only a dose of 10,0 mg/kg of compound 3 reached more than 50,0 % of inflammation inhibition (65,7 %).
Since the structures of compounds 2 and 3 are almost the same, only differentiated in C-3, we have suggested that the increase in anti-inflammatory activity for triterpene 2 is due to the presence of a carbonyl group in C-3 in its structure instead of the hydroxyl group found in triterpene 3.
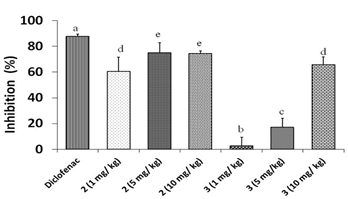
Fig. 3 Anti-inflammatory effects of different doses of compounds 2 and 3 in Croton oil- induced ear edema in mice. Different letters represent significant differences (p < 0.05)
Compounds 1, 3 and 4 as well as betulin 15 have previously been proven to have anti-inflammatory properties. Herein we have demonstrated that compound 2 has also the same biological activity.
We consider that their presence in the bark of M. elaeodendroides plays an important role in the anti-inflammatory activity found in the extract of petroleum ether /diethyl ether (1:1).
Conclusions
In this work we were able to obtain a petroleum ether /diethyl ether (1:1) extract from the bark of Maytenus elaeodendroides Griseb. For the first time, four triterpenoid compounds (lupane series, lupene-type) were isolated. Those were: 28-hydroxy-3-oxo-20(29)-lupene; 16-hydroxy-3-oxo-20(29)-lupene; 3dihydroxy-20(29)-lupene and acid 3-oxo-20(29)-lupene-28-oic. Particularly, 16-hydroxy-3-oxo-20(29)-lupene was isolated for the first time from Celastraceae family. At certain doses the obtained extract showed an anti-inflammatory response as potent as that one elicited by diclofenac (reference drug). This is the first time it is reported an anti-inflammatory activity for compound 2 (16-hydroxy-3-oxo-20(29)-lupene). Although lower than that of diclofenac, it exhibited a still high effect. We have suggested that an increase in its anti-inflammatory activity is likely associated to the presence of a carbonile group in C-3















