Introduction
Ionic liquids are an interesting type of compounds due to its several applications. There are reports of ionic liquids in synthesis 1, green synthesis of biodiesel using ionic liquids 2, ionic liquid assisted cellulose aerogels for cleaning an oil spill 3, and a facile preparation of a lignocellulose aerogel from a solution of wood in an ionic liquid by cyclic freeze-thaw process.4 Also, some authors report application as dual catalyst-solvent 5, in upgrading of heavy crude oils.6 Recently, other important works as reviews had been written about ionic liquids.7
Their physicochemical properties can be readily tuned for specific applications by utilizing different combinations of the anions and cations. Ionic liquids therefore possess unique physicochemical properties namely, a negligible vapor pressure, a high thermal stability, a high ionic conductivity, and a wide electrochemical window.8
The term ionic liquid has been reserved to designate those compounds that are formed exclusively by ions and at moderate temperatures are liquids, where its upper limit can be considered to be of the order of 100 °C. There are references of this type of compounds since 1914 but it is in the second half of the twentieth century when F.H. Hurley and T. P. J, Wier discover that salts that are liquid at room temperature can be synthesized.9
In these few decades in which the synthesis of ionic liquids has been worked on, the necessary temperature has been reduced to achieve a liquid formed by ions from 800 °C, which is the melting temperature of sodium chloride to temperatures below - 90 ºC /10/. Using different cation and anion precursors, a large number of compounds are obtained whose characterization shows the great variety of properties they offer.10,11 Some of the cations that have been most used in obtaining ionic liquids are those that have the imidazole ring as the nucleus. The imidazole nucleus is found to be part of the main structures of the components of human beings such as the amino acid histidine, Vit-B12 and in the DNA bases and puric structures.12 It is present in the structure of many molecules, whether natural or synthetic drugs such as metronidazole, miconazole, clotrimazole, ketoconazole, among others.
The research group "Bioactive Compounds and Sustainable Chemistry", of the Department of Chemistry of the University of Oriente, in Cuba, has dedicated part of its efforts to study these compounds. As results there are several families of imidazoles synthesized by different methods. In this way some groups of 4,5-disubstituted, 2,4,5-trisubstituted and 1,2,4,5-tetraaryl substituted imidazoles have been obtained.13-15
2,4,5-trisubstituted imidazoles were evaluated as potential antifungal agents for agriculture using theoretical studies (QSAR / QSPR) 16 in addition, a series of such tetra-aryl substituted imidazoles are being studied, as potential drugs against lehismaniasis.17
Many scientific literatures report the synthesis of ionic liquids based on substituted imidazoles 18, but no reports of the use of tetra-aryl imidazoles substituted for this purpose have been found. Based on the foregoing, the purpose of this investigation is to conduct a preliminary study, to obtain ionic liquids from 1,2,4,5-tetraaryl substituted imidazoles, as a new possible application for these compounds.
Methods and materials
All the reagents used were pure for synthesis. From Uni-Chem Company (China) boric acid, nitric acid, ammonium acetate, dichloromethane, hexane, diethyl ether, toluene, acetonitrile and benzene were used. From Merck (Germany) p-nitrobenzaldehyde, m-toluidine and n-butyl bromide were used.
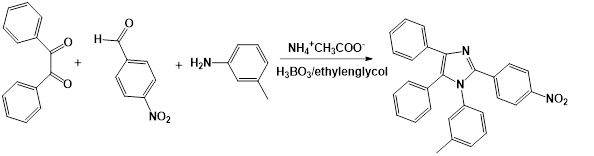
Oxidation of benzoin to benzyl (Fig 1)
0,071 mol of benzoin and 1,034 mol of concentrated nitric acid (HNO3) were added in a balloon coupled to a reflux condenser. The mixture was heated to reflux in a water bath, until the evolution of the nitrogen oxides ceased (approx. 45 min). It was allowed to cool and the reaction mixture was poured into a beaker with cold water, stirred until the oil completely became a yellow solid (benzyl). The product was filtered and the purification was carried out by recrystallization of ethanol. The benzyl obtained after recrystallization was a bright yellow crystalline solid with the form of needles. The melting temperature was 93,8- 94,9 °C, which is in correspondence with the literature (94,78 ° C).19 Yield: 79 %.
Synthesis of 1-(3-methtylphenyl)-2-(4-nitrophenyl)-4,5-diphenylimidazol
0,035 mol of p-nitrobenzaldehyde, 0,035 mol of benzyl and 0,035 mol of the aromatic amine m-toluidine, 0,0035 mol of boric acid (H3BO3), were added in a flask fitted with a reflux condenser. Later 0,028 mol of ammonium acetate (NH4 +CH3COO-) and 105 mL of ethylene glycol, were added. The mixture was heated under reflux until 10 min after the appearance of a precipitate in the reaction medium and allowed to cool to room temperature. It was then added in a beaker with 100 mL of water. Yield: 47 %. 1H-NMR (500 MHz-MeOD): δ= 2,21 (2H, CH3); 7,33-7,49 (4H, CHAR); 8,17-8,19 (4H, CHAR); 8,29-8,32 (4H, CHAR). The compound is also characterized by ESI-MS.
Quaternization reaction of imidazoles
0,0023 mol of 1-(3-methylphenyl)-2-(4-nitrophenyl)-4,5-diphenylimidazol and 0,004 mol of C4H9Br with 0,23 mL of toluene were added on a round bottom balloon. Heated under reflux for 30 h, 2 layers formed. The bottle was cooled to room temperature and then allowed to cool overnight. A solid formed and the excess of toluene was decanted. The product was recrystallized with a minimum amount of acetonitrile, with the addition of 1mL of diethyl ether. It was then filtered under vacuum and the resulting solid dried for 48 h in a desiccator. Yield: 53 %. The quaternary imidazole salt was characterized by ESI-MS.
Thin layer chromatography
Thin layer chromatography was performed on the already recrystallized products. A mixture of solvent benzene-dichloromethane-hexane (2: 2: 1) was used as the mobile phase and Silica Gel-60 plates with aluminum support as the stationary phase. For the development of the plates, a UV lamp was used.
Mass Spectrometry by Electrospray ionization (ESI-MS)
Mass spectra were obtained on a Synapt G2 HDMS Quadrupole Acceleration Mass Spectrometer, Waters, Milford, MA (USA), by positive ionization with a resolution of 15 000 (FWHM). Leucine enkephalin was used as the blocking mass. Acetonitrile was used as a solvent.
Results and discussion
Benzoin oxidation to benzyl was carried out as experimental part description, obtaining a bright yellow crystalline solid as needles.19 The yield is similar to reported in the bibliography.13 In order to check the purity and as criteria for the formation of benzyl, after recrystallization, a CCF was performed. The benzoin and benzyl were punctuated simultaneously on the same chromatographic plate as seen in figure 2.
Table 1 shows the Rf that were calculated for each product. The appearance of two spots was observed when the chromatographic plaque was revealed, one corresponding to benzoin and a smaller one at the same height as the benzyl, which is inferred that the benzoin presented as an impurity to the benzyl. In the case of benzyl, a single spot was observed, evidence that the reaction occurred in its entirety.
After benzoin oxidation, the synthesis of the 1,2,4,5-tetraaryl substituted imidazol was carried out, as experimental part describes (figure 3).
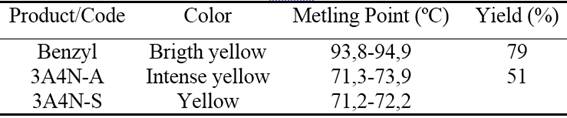
The color, reaction time, melting temperatures and synthesis yields of the 1,2,4,5-tetraaryl substituted imidazol are reported in table 2.
When the purification of the product was carried out by recrystallization, the formation in the filtrate of an oily phase more intense coloration than the solution phase was observed. Once resting, both phases were crystallized. Those crystals were filtered under vacuum and placed in the desiccator for 48 h.
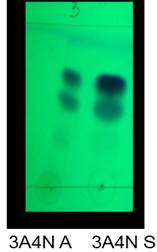
A second recrystallization was performed for which a separating funnel was used to hot isolate the oily phase from the solution. The crystals were filtered under vacuum and placed in desiccator for 48 h. For subsequent analysis (see table 2), it should be taken into account that for each product of the imidazole synthesis, two fractions were obtained which were denoted as A (solid obtained from the oil) and one S (solid obtained from the solution). Tin layer chromatography was used for imidazol purity criteria too (figure 4). The formation of two spots was observed.
When performing a simultaneous analysis of the two isolated fractions of the imidazol obtaining reactions, it can be observed that the spots with Rf-1 presented the same value for the two fractions, the same behavior is the one observed for spot 2 with Rf-2. If the values of Rf-2 are analyzed and compared with the Rf-2 obtained for benzyl, it can be observed that it is the same, so it can be ensured that this impurity corresponds to mentioned compound, which is understandable because it is a common starting reagent for all synthesized imidazoles. Each of the solids obtained for the product of the synthesis of the imidazoles was tested with 2,4-DNFH and an orange precipitate was obtained, which shows the presence of the carbonyl group. This result corroborates that imidazoles are doped with benzyl.
In this way, we can infer that the two fractions have similar chemical composition from the chromatographic point of view, and it was not necessary to have them isolated. When analyzing the chromatographic plates, it is possible to notice that the synthesized imidazoles can be positional isomers, so there are no significant differences in their polarity and therefore all Rf are very similar.
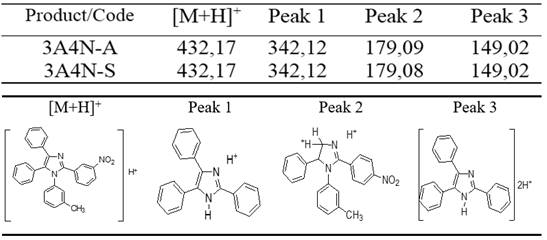
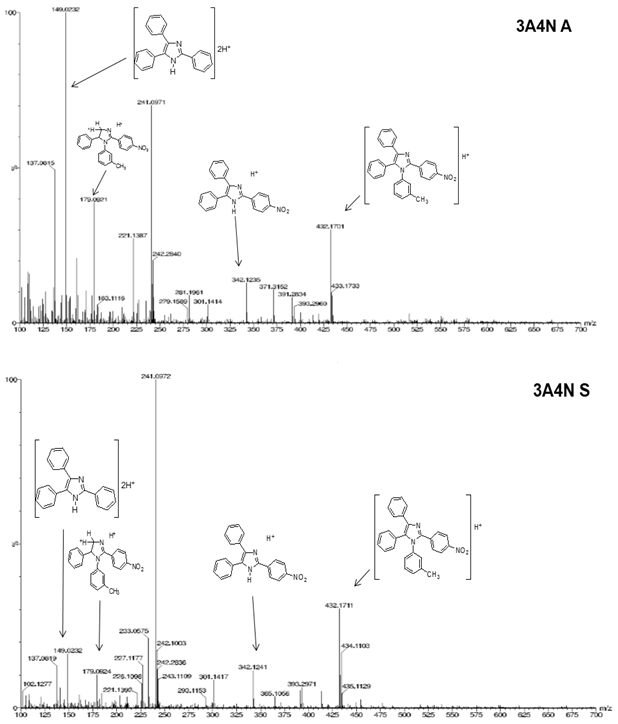
To have a clearer idea of the structure of the imidazoles obtained, as illustrated in the title of this article, an Electro Spray Ionization Mass Spectrometry (ESI-MS) analysis was performed. This allowed corroborating the obtaining of this imidazole in the fractions described above (3A4N-A and 3A4N-S) and that whose spectra coincide almost completely. table 3 shows the main peaks found and predicted in the fragmentation scheme (figure 5).20 First of all, it was possible to identify the M+H peak, which shows coincidence with the molar mass of 1-(3-methylphenyl)-2-(4-nitrophenyl)-4,5-diphenylimidazol (table 3). Some peaks corresponding to defined fractionations for this imidazole were then identified, as shown in figure 5 and figure 6.
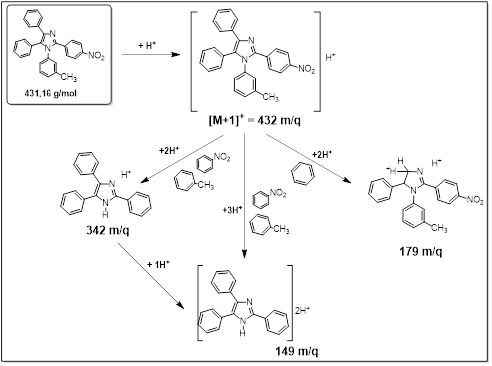
Fig. 5 ESI-MS fragmentation scheme proposed for 1-(3-methylphenyl)-2-(4-nitrophenyl)-4,5-diphenylimidazol
The product of the formation reaction of 1-(3-methylphenyl)-2-(4-nitrophenyl)-4,5-diphenylimidazole was used to the quaternization reaction with n-butyl bromide (Yield: 53 %). The reaction was carried out at reflux temperature that was considered sufficient for the n-butyl bromide to react.11 Because Br- is a good outgoing group, although perhaps this was not enough because the substituents of the imidazole ring are not electrodonors which means that the N-3 is not basic enough. It is also important to consider the steric limitation around N-3 by bulky substituents. Therefore, such a reaction may need to be carried out in subsequent experiments at a much higher temperature, with the use of microwave energy or with phase exchange catalyst catalysts. These conditions allow to achieve the correct course of the reaction.
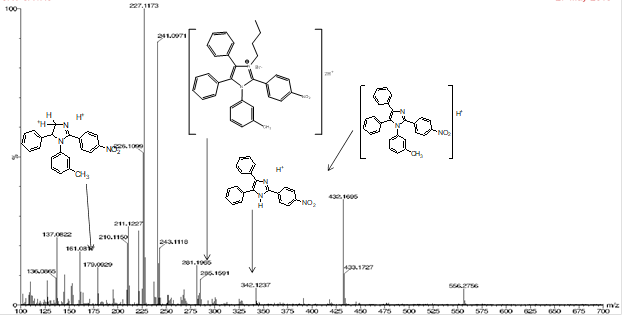
As is known, an indispensable condition for a compound to be an ionic liquid is the melting temperature value which must be below 100 °C. Often, the salt obtained as a product of the quaternization reaction already has moderate melting temperatures. In this case the product obtained from the quaternary salt synthesis reaction has melting point below 100 °C (Mp = 73,7-79,2 °C). Although the values are very close to the tetra-substituted imidazol, it is possible to suspect about a new compound. For clarity, it was necessary to registry ESI-MS spectra for quaternary imidazol salt (figure 7).
In this case, many peaks are exactly the same observed for imidazol 3A 4N. Nevertheless, a new peak appears in 285 m/q, probably corresponding to biprotonated specie of quaternary salt. Also, there is a new peak at 556 m/q respect to 3A 4N, that can be associated to this new compound as well (figure 8) 20.
Conclusions
This ESI-MS study allowed us to monitor the synthesis of an imidazole as well as a derived quaternary salt using this technique. The ESI-MS spectra for 1-(3-methylphenyl)-2-(4-nitrophenyl) -4,5-diphenylimidazol indicates the viability for this method of imidazol synthesis by the use of boric acid as catalyst. ESI-MS shows for the quaternary salt of this imidazole, the presence of a peak that suggest the existence of 1-(3-methylphenyl)-2-(4-nitrophenyl)-3-N-buthyl -4,5- diphenyl imidazol bromide. Melting points indicates the possibility of ionic liquids presence.