Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.26 n.1 La Habana ene.-mar. 2009
RESEARCH
Improving the expression of Human Epidermal Growth Factor in Saccharomyces cerevisiae by manipulating culture conditions
Incremento de la expresión del Factor de Crecimiento Epidérmico en Saccharomyces cerevisiae mediante
la manipulación de las condiciones de cultivo
Jorge Valdés1, Ernesto Mantilla1, Gabriel Márquez1, Regla M Bonilla1, Victoria M Lugo1, Mariela Pérez1, Yanara García1, Emilio Narciandi2
1Technological Development Unit
2Transfer Technology Department
Center for Genetic Engineering and Biotechnology, CIGB. Ave. 31 / 158 and 190, Playa, PO Box 6162, Havana 10600, Cuba
ABSTRACT
Human Epidermal Growth Factor (hEGF) is a protein molecule with potent mitogenic activity, increasing the rate of wound and ulcer healing in different tissues of the human body. In recent years, the Center for Genetic Engineering and Biotechnology (CIGB) has carried out projects at the developmental stages for the application of hEGF in novel therapies. It is now necessary to increase production capacity within the same facility, to meet the increasing demand of this product.The aim of this work was to increase hEGF expression levels by optimizing the culture conditions. The culture media, pH and temperature dependency of hEGF expression and degradation rate were studied. An increase of 1.8 times of the hEGF expression level was achieved by adding to the medium 50 mg/L of histidine and leucine. A factorial design experiment was applied to optimize the yeast extract and bacteriological peptone concentration in the culture media. The statistical analysis of response surface predicted that using 18 g/L of yeast extract and 25 g/L of bacteriological peptone improved the hEGF expression level up to 3.5 mg/g. This is the highest expression level reported for hEGF in yeast up to now. Furthermore, the form in which the hEGF degradation rate was reduced to less than 10% in fermenters when the culture was made on the optimized medium at pH 6.5 and 25 °C, is discussed.
Keywords: Culture media, expression improvement, human epidermal growth factor, proteolysis control, Saccharomyces cerevisiae.
RESUMEN
El Factor de crecimiento epidérmico humano (FCE-h) es una proteína que posee una potente actividad mitógenica la cual se manifiesta en el incremento la velocidad de cicatrización de heridas y ulceras en diferentes tejidos del cuerpo humano. Durante los últimos años el Centro de Ingeniería Genética y Biotecnología (CIGB) ha estado involucrado en diversos proyectos de terapias novedosas asociadas al uso del FCE-h. Actualmente existe la necesidad de aumentar de forma significativa la capacidad productiva de esta molécula en la misma instalación industrial para dar respuesta a la creciente demanda de este producto. El objetivo central de este trabajo fue el aumento del nivel de expresión de FCE-h mediante la optimización de las condiciones de cultivo. En el siguiente trabajo se estudió la influencia del medio de cultivo, el pH y la temperatura sobre la expresión del Factor de Crecimiento Epidérmico humano (FCE-h). Se logró un aumento de 1.8 veces en el nivel de expresión del FCE-h mediante la adición de 50 mg/L de histidina y leucina respecto al medio base. El medio de cultivo fue optimizado empleando un experimento factorial de superficie de respuesta. Se encontró un óptimo de concentración de extracto de levadura y peptona bacteriológica de 18 y 25 g/L, respectivamente, correspondiéndose con un nivel de expresión máximo de 3.5 mg/g. Este nivel de expresión es el máximo reportado para el FCE-h en levaduras. Por otra parte, se discute como es posible lograr un control de la proteolisis del FCE-h cuando se opera a un pH de 6.5 y una temperatura de 25 °C.
Palabras clave: Medio de cultivo, optimización, mejora de la expresión, factor de crecimiento epidérmico humano, control de la proteolisis, Saccharomyces cerevisiae.
INTRODUCTION
The human Epidermal Growth Factor (hEGF) is a 53 aa. single-chain polypeptide that stimulates the proliferation of a number of cell types including epithelial and epidermal tissues and is also capable of inhibiting gastric acid secretion in man (1). These properties have led to its use in a number of therapeutic applications such as wound healing, healing of corneal surface abrasions and transplants, the treatment of pressure and diabetic neuropathic foot ulcers, among others (2-4).
The biotechnological production process is the result of time-consuming and expensive research and development. For optimal returns, it is necessary to improve the producer strain, the culture medium and other process parameters to reach a maximal productivity or the highest production capacity. At our institution extracellular hEGF was expressed in Saccharomyces cerevisiae and Pichia pastoris, obtaining the best overall productivity in S. cerevisiae, for which reason it was selected as the production host (5).
Genetic characteristics and environmental variables play an important role on the productivity of biotechnological processes. Recombinant protein production through its host is affected by many environmental variables, such as the culture media, pH, temperature, and shaking and aeration rate, among others. In general, many studies have been carried out on the effects of media composition on cell growth and protein expression (6-10), as well as on protein degradation during the cell culture process (11-13) due to the cosecretion of proteases or their release through cell lysis.
In this context, a number of adjustments to the standard recombinant yeast fermentation process have been reported, including changing the culture pH or the temperature (11, 14-17).
The expression of hEGF has been thoroughly studied in the past as a model of a protein expression in yeast, however the reported yield and expression level is quite low, from 0.5 to 2.5 mg/g (11, 18). Up to now there is no evidence in the literature on a systematic approach to study the culture media and operational bioreactor variables to improve hEGF expression. Besides, no papers have been devoted to the development of high productivity-large scale hEGF processes for commercial use.
The present paper examines the results of a preliminary study for the development a large scale hEGF production process, specifically focused on the analysis of the effect of media composition as well as the influence of temperature and pH on hEGF expression and degradation rate during the culture process.
MATERIALS AND METHODS
Chemicals
All chemicals were of spectral or analytical grade and unless otherwise stated, all chemicals used for preparing the culture media and buffers were obtained from Merck KGaA (Darmstadt, Germany), and Biocen S.A. (Havana, Cuba).
Microorganism
The recombinant producer strain Saccharomyces cerevisiae UD05PS102 (MATa, leu2-3- 112, his4-519) was obtained and preserved by CIGB culture collection (5). The pHEGF27 expression plasmid complemented host strain mutation in the ura3 gene.
Culture media and conditions
Two milliliters of thawed cell suspension were inoculated into the 400 mL G0 seed medium (5) in a 2-L flask. This seed culture was incubated at 28 °C and 150 rpm for 48 h. For the shake flask experiments, 5 mL of this seed culture were transferred to 100 mL of the production medium, and for fermentation studies the entire 400 mL were inoculated.
The production media composition for growth and hEGF expression cultures are YEPS1 (sucrose 40 g/L, bacteriological peptone 20 g/L, yeast extract 10 g/L, pH 6) and YEPS3 (sucrose 40 g/L, leucine 0.9 g/L, histidine 0.31 g/L, NH4Cl 1 g/L, MgSO4·7H2O 0.9 g/L, KH2PO4 1.4 g/L, NaCl 0.035 g/L, CaCl2·2H2O 0.175 g/L, trace solution 1.5 mL/L, vitamin solution 3.5 mL/L, pH 6). The bacteriological peptone and yeast extract concentrations ranged from 5 to 25 g/L. The preparation of trace and vitamin solutions were described previously (5).
All shake flask studies were performed in 1 L flasks at 28 °C and 150 rpm for 48 h in YEPS1 or YEPS3, depending on the experiment. Experimental studies in the medium named 2X, 1X, 0.5X, 0.25X correspond to 2, 1, 0.5 and 0.25 times the YEPS1 medium, respectively.
For fermentation studies the culture was in a fully-automated 7-L fermenter (BE Marubishi, Tokyo, Japan). Each bioreactor was loaded with 4.0 L of the YEPS1 or YEPS3 media supplemented with amino acids (leucine and histidine). Unless otherwise stated the fermentation parameters were pH 5.0, 30 °C, 700 rpm and 1.0 vvm, operated in a batch-wise mode with up to 22 h of culture time.
Culture media was optimized in the shake flask experiments, specifically, the concentration of bacteriological peptone, yeast extract, leucine and histidine. Fermentation pH and temperature were studied on bioreactors.
The hEGF stability study of the culture supernatant was carried out in 1.5 mL vials at various pH and temperatures.
Analytical determinations
Cell mass determinations
Each sample (~10 mL) was centrifuged and supernatants were frozen at -20 °C until later used in ELISA and RP-HPLC analyses. The biomass was determined gravimetrically (wet weight) and converted to dry weight (dw) by an in-house established correlation between dry and wet-weight concentrations where: 1 g/L dw = (0.280 ± 0.007) g/L wet weight (hereafter: average ± standard deviation).
Determination of hEGF concentration
hEGF concentration was determined using an ELISA against a secondary standard of human Epidermal Growth Factor. The procedure was performed as described in a previous paper (5).
The quantification of hEGF degradation was performed by RP-HPLC in a Merck-Hitachi analytical system (Merck KGaA, Darmstadt, Germany), employing a C8 column (208TP5415; Ø 4 x 150 mm, 5 mm) from Grace Vydac (Hesperia, CA), keeping the columns at 37 °C. A gradient from 20 to 40% of buffer B in 50 min at a flow of 0.8 mL/min was employed. Buffers A and B were 0.1% (v/v) of trifluoroacetic acid, and acetonitrile (Acetonitrile-212, Caledon Laboratories Ltd., Georgetown, ON, Canada) plus 0.05% (v/v) of trifluoroacetic acid, respectively.
hEGF stability studies in the culture supernatant
For temperature hEGF stability studies in the culture supernatant, a sample from the fermentation harvest (at pH 6.5) was centrifuged (4000 g, 20 min) and incubated at 4, 28 or 37 °C. Each sample was assayed at 12, 24 and 48 h.
Similar studies were done for pH stability studies adjusting the supernatant to different pH (5.0, 5.5, 6.0, 6.5, 7.0, 7.5 and 8.0) with 1 M of NaOH or HCl and incubated for 24 h at 28 °C.
The degree of hEGF degradation was measured by RP-HPLC through the quantification of the relative hEGF peak area.
Statistics, experimental design
A factorial experimental design 32 (surface response) was applied to optimize the concentration of bacteriological peptone and yeast extract in the YEPS3 medium. Both variables were tested in three coded levels: low (-1), medium (0), high (+1). The coded values corresponded for both variables as follows: -1 (5 g/L), 0 (15 g/L), + 1 (25 g/L). Once the hEGF expression level was determined (as the ratio of hEGF to biomass concentration), a second order polynomial model was fitted to the response data obtained from the design. All the statistical procedure for the experimental design, data analysis and optimization were performed with the software package Statgraphics Plus® for Windows 5.1.
RESULTS AND DISCUSSION
Effect of the leucine and histidine supplement in the culture media
The effect of the concentration factor of peptone and yeast extract on hEGF expression in YEPS1 is shown in figure 1. As mentioned before, the producer strain carries mutations for the synthesis of histidine and leucine, making it necessary to add both amino acids to the culture medium. The inclusion of bacteriological peptone and yeast extract in the culture medium works as a source of amino acids, vitamins, nucleotides and trace elements. We believe that yields may be enhanced when precursors such as amino acids are added in a manner that considers both the strains auxotrophy and the primary amino acid composition of the protein produced.
It can be observed that when the concentration of peptone and yeast extract are doubled, the expression does not increase, but when the concentration of both components are diluted 1/2 or 1/3 the expression drops 3-fold compared to the standard culture conditions (Figure 1).
This result indicates that the concentration range of 0.5 to 1.0 X in YEPS1 is suitable for further studies on media development. In fact at this range of bacteriological peptone and yeast extract concentration (0.5 to 1 X of YEPS1 media) we studied the amino acids supplements of leucine and histidine (Figure 2). It is observed that the addition of both amino acids increases hEGF expression 1.7-2.0 times for both concentration conditions (Figure 2).
It is well known that overproduction of recombinant proteins usually diminishes the availability of free amino acids (7) and therefore the replenishment with the necessary nutrients results in a substantial increase in protein production. Although part of the leucine and histidine needs for yeast growth is provided by complex components of the medium (as bacteriological peptone and yeast extract) hEGF expression is limited. Leucine is the third most frequent amino acid in the hEGF protein (9.2%) as well as in S. cerevisiae whole protein yeast (8.3%), while the composition of the leucine in the complex medium is of about 3.8% to 6.5% (10).
In the case of histidine the possible limitation is very severe. The proportion of histidine in the hEGF protein is 4.3%, but in the whole protein yeast it is 2.2%. On the other hand, the composition of histidine in the complex media is very low (0.7% to 1.7%). This demonstrates that the addition of both amino cids significantly improves the nutritional balance of the culture medium, thereby achieving an increase in hEGF expression.
Optimization of bacteriological peptone and yeast extract concentration in the YEPS3 medium
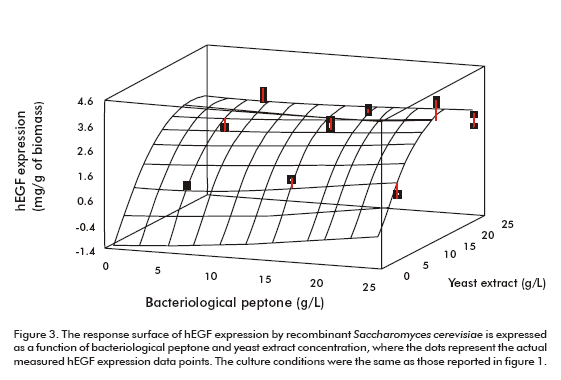
Peptone and yeast extract concentrations were optimized using a medium already supplemented with both amino acids as explained above. Figure 3 shows the surface response of the optimization experiment. According to the optimization program the highest hEGF expression level (3.33 mg/g of biomass) could be obtained for the optimum values of 25.0 g/L and 17.7 g/L of bacteriological peptone and yeast extract, respectively (Figure 3).
The procedure applied for optimizing the culture medium produced an increase of 3 times the expression level of hEGF (3.22 ± 0.20 mg/g of biomass). This optimized medium has been proven in batch fermentation with bioreactors (Table 1).
Even when the YEPS3-optimized medium seems to perform better in bioreactors in terms of hEGF expression (2.60 ± 0.31 vs 1.20 ± 0.13 mg/g of biomass) the yield was lower than expected (only 2.2 times higher than for YEPS1 expression). On the other hand, hEGF degradation was lower in the YEPS3 medium (1.7 times) than in YEPS1, but unfortunately degradation is still at a considerable level (25%).
It is well known that the extent of degradation of a secreted protein (including the hEGF) depends on the cultures pH, temperature, culture time, cell concentration, among others (16). Cell concentration at 36 h was 1.8 times higher in the YEPS3 medium than in YEPS1 (data not shown) and this fact could also contribute to the increase in degradation rate and consequently reduce overall hEGF expression level. This analysis indicated that other fermentation variables could contribute to that fact that the optimum hEGF expression level obtained in shake flask experiments was not reached.
Influence of temperature and pH on hEGF expression in the YEPS3-optimized medium
Figure 4 shows a stability study of the hEGF culture supernatant at different temperatures and pH. As expected the low temperature decreased hEGF degradation rate throughout the range tested (Figure 4A), but after 24 h of incubation there was practically no difference observed between 28 and 37 °C, which means that temperature itself is not enough to control hEGF proteolysis (Figure 4).
In the pH study (Figure 4B), at 6.5 there is a significant decrease of hEGF proteolysis (for 24 h incubation at 28 °C) compared to the range of pH of 5.0-6.0, and, at pH above 7.0, hEGF proteolysis is controlled (only 20% of the hEGF is degraded).
The results of the control of hEGF degradation by manipulating the pH are similar to those reported by Clare et al. (11, 12) and Hong et al. (16), due to a decrease of yeast proteolytic activity. According to Jahic et al. (14, 15) is it advisable to operate the fermentation process in the range of 20 to 25 °C in order to reduce cell lysis occurring during fermentation, and therefore decrease the proteases released in the culture medium. Based on these findings and our previous experience, we decided to establish the culture parameters for hEGF fermentation at 30 to 25 °C and with pH from 5.0 to 6.5.
Figure 5 shows the hEGF expression and degradation profile using the new culture conditions. The hEGF expression was of 3.50 ± 0.32 mg/g, which is similar to the expression obtained in the shaking flask experiments, and the hEGF degradation was almost completely controlled at less than 10%. This expression level is significantly higher than the maximum value reported up to now by Coppella and Dhujarti (18) for hEGF in S. cerevisiae (0.52 mg/g) and in P. pastoris (11) (2.54 mg/g). It is important to point out that the yield observed is a function of the expression rate minus the degradation rate, and therefore an engineering approach to the optimization of the bioprocess is to maximize the expression and minimize proteolysis at the same time (Figure 5).
The decision for establishing the operation pH at 6.5 is based on the common practice at a production scale to operate with the pH as low as possible so as to reduce the risk of contamination, but the authors agree that it will be interesting to explore in the future, the optimum pH for maximizing hEGF expression in this particular process.
CONCLUSIONS
Optimizing the bacteriological peptone and yeast extract concentration with a supplement of leucine and histidine, has a significant impact in increasing recombinant hEGF protein expression. The optimized YEPS3 culture medium produced a 3-fold increase in hEGF expression. On the other hand, it was demonstrated that changing the culture conditions to pH 6.5 and 25 °C using the optimized YEPS3 it is possible to control hEGF proteolysis in fermenters, ensuring the operation of the entire fermentation process at a maximum hEGF expression. This is the first report where a combination of the culture medium, temperature and pH is able to improve hEGF expression and control proteolysis. The culture protocol will be the milestone for the future improvement of a large-scale high efficiency hEGF fermentation process.
REFERENCES
1. Nakagawa S, Yoshida S, Hirao Y, Kasuga S, Fuwa T. Biological effects of biosynthetic hEGF on the growth of mammalian cells in vitro. Differentiation 1985;29:284-8.
2. Carpenter G, Cohen S. Epidermal growth factor. Ann Rev Biochem 1979;48:193-216.
3. Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem 1990;265:7709-12.
4. Lee DN, Kuo TY, Chen MC, Tang TY, Liu FH, Cheng CF. Expression of porcine epidermal growth factor in Pichia pastoris and its biology activity in early-weaned piglets. Life Sci 2006;78:649-54.
5. Cinza AM, Quintana M, Lombardero J, Poutou R, Pérez E, Pérez LC et al. Establecimiento de un cultivo discontinuo para la producción de factor de crecimiento epidérmico humano en levaduras. Caracterización del producto. Biotecnol Apl 1991;8:166-73.
6. Nowruzi K, Elkamel A, Scharer J, Cossar D, Moo-Young M. Development of a minimal defined medium for recombinant hIL-3 production by Streptomyces lividans 66. Biotechnol Bioeng 2008;99:214-22.
7. Chen PT, Chao YP. Enhanced production of recombinant nattokinase in Bacillus subtilis by the elimination of limiting factors. Biotechnol Lett 2006;28:1595-600.
8. Görgens JF, Passoth V, van Zyl W, Knoetze JH, Hanh-Hägerdal B. Amino acid supplementation, controlled oxygen limitation and sequential double induction improves heterologous xylanase production by Pichia stipitis. FEMS Yeast Res 2005;5:677-83.
9. Wang ZW, Chen Y, Chao YP. Enhancement of recombinant protein production in Escherichia coli by coproduction of aspartase. J Biotechnol 2006;124:403-11.
10. Hahn-Hagerdal B, Karhumaa K, Larsson C, Gorwa-Grauslund M, Gorgens J, van Zyl W. Role of cultivation media in the development yeast strains for largescale industrial use. Microb Cell Fact 2005;4:1-16.
11. Clare JJ, Romanos MA, Rayment FB, Rowedder JE, Smith MA, Payne MM, et al. Production of mouse epidermal growth factor in yeast: high-level secretion using Pichia pastoris strains containing multiple gene copies. Gene 1991;105:205-12.
12. Clare J, Scorer C, Buckholz R, Romanos M. Expression of EGF and HIV envelope glycoprotein. Methods Mol Biol 1998;103:209-25.
13. Hong F, Meinander NQ, Jönsson LJ. Fermentation strategies for improved heterologous expression of laccase in Pichia pastoris. Biotechnol Bioeng 2002;79:438-49.
14. Jahic M, Gustavsson M, Jansen AK, Martinelle M, Enfors SO. Analysis and control of proteolysis of a fusion protein in Pichia pastoris fed-batch processes. J Biotechnol 2003;102:45-53.
15. Jahic M, Wallberg F, Bollok M, García P, Enfors SO. Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb Cell Fact 2003;2:6-16.
16. Hong S, Moon H, Kim H, Rhee S, Choi E, Kim I. Optimal strategy of pH control in the production of recombinant human epi-dermal growth factor by Hansenula polymorpha. Process Biochem 2002;38:487-95.
17. Ohya T, Morita M, Miura M, Kuwae S, Kobayashi K. High-level production of prourokinase-annexin V chimeras in the methylotrophic yeast Pichia pastoris. J Biosci Bioeng 2002;94:467-73.
18. Coppella S, Dhujarti P. A mathematical description of recombinant yeast. Biotechnol Bioeng 1990;35:359-74.
Received in September, 2008.
Accepted for publication in March, 2009.
Jorge Valdés. Technological Development Unit. Center for Genetic Engineering and Biotechnology, CIGB. Ave. 31 / 158 and 190, Playa, PO Box 6162, Havana 10600, Havana, Cuba. E-mail: jorge.valdes@cigb.edu.cu