INTRODUCTION
Life and career (Passy, 1890; Anonymous, 2023)
Jean-Augustin Barral (Figure 1) was born in Metz (Moselle, France) on January 30, 1819, the only son of Jeanne Françoise Remy and Jean Augustin Barral, a former soldier in the Napoleon army, who after the Bourbon Restoration retired to Metz in order to live with his modest pension. Barral took his basis education at the Collège Royal de Metz and the Collège Sainte-Barbe in Paris. In 1838, at the age of 19, he entered the École Polytechnique from where he graduated in 1840 to become attached as tobacco engineer to the scientific staff of the Manufacture Royale de Tobacco of Paris (the French tobacco monopoly). While in this position he succeeded in extracting nicotine form the leaves of the tobacco plant, determine its composition and formula and demonstrate experimentally its poisonous effects. Between 1841 and 1852 served as professor of physics and chemistry at the Collège Saint-Barbe and in 1845 as répétiteur of the chemistry course given by Victor Regnault (1810-1878) at the École Polytechnique. The first part of his academic life was devoted to inorganic and organic chemistry subjects and the second to problems related to agriculture, irrigation, and fertilizers.
Barral took an active part in the public and political affairs of France in the tumultuous years of his life (including a short time in jail). His prolific research activities were rewarded by many scientific and public organizations. In 1856 he became member of the Société Nationale d'Agriculture de France (physics and chemistry section) and in 1871 was elected its permanent secretary. In 1863 he was appointed chevalier of the Legion d'Honneur, promoted to officier in 1863 and to commandeur in 1880.
Barral took a strong participation in all the activities related to physics of the atmosphere. On June 29 and July 17, 1850, Barral, together with Jacques Alexander Bixio (1808-1865), performed two flying balloons climbs, the fall of the balloon in first one almost ended in a tragedy. Measurements of the outside temperature showed that the temperature of the different upper layers of the atmosphere changed in a similar manner as the ones near the surface. In 1862 Barral, together with his friend Gaspar-Felix Tournachon (Nadar, 1820-1910) participated in the creation of the Société de Navigation Aérienne and in 1863 he was elected president of the same. In 1853, Barral, Agénor de Gasparin (1810-1871), Émilien Renou (1815-1902), Charles Sainte-Claire Deville (1814-1876) and others, founded the Société Météorologique de France. Barral was member of the Société d’Encouragement à l’Industrie Nationale, Société Internationale d’Études Sociales, Société des Agriculteurs de France, Conseil Général de la Moselle, etc. He participated in the creation and direction of several scientific, political and social journals, among them Journal d'Agriculture Pratique, Journal de l'Agriculture, La Démocratie Pacifique, Cercle de la Presse Scientifique, Dictionnaire d’Agriculture, La Presse Scientifique des Deux Monde, etc. In 1857 he received a medal from the Société Nationale d’Agriculture and in 1863 the Académie des Sciences awarded him the Morogues Prize for his efforts in the promotion of agricultural science. Jean-Augustin Barral is one of the 72 French scientists whose name is inscribed on the first floor of the Eiffel Tower.
Barral died at Fontenay-sous-Bois, near Paris, on September 10, 1884.
Scientific contribution
Barral wrote more than 70 papers and books (i.e., Barral, 1850b, 1858, 1876, 1878, 1884) about inorganic, organic, and biochemistry, agriculture, irrigation, physiology, toxicology, etc. As customary for candidates to the French scientific academies, he published a booklet describing the results of his scientific research (Barral, 1849d). In addition to the subjects described below he studied the difference between mercury chlorides and the electrochemical gilding materials (Barral, 1847b); wrote a diary of his trips in a flying balloon (Barral and Bixio, 1850); the guano of Peru (Barral, 1862, 1874); the influence of underground humidity and soil capillarity on the growth of vineyards (Barral, 1883); cleaning processes for foreign matter in wool (Barral, 1875; Barral and Salvetat, 1875); analysis of sugar pulps obtained by different procedures (Barral, 1879); analysis of the food intake of a wood pigeon (Barral, 1881a); nitrogenous food required by certain animals (Barral, 1881b); the phylloxera disease in vines (Barral, 1882a), etc.
Nicotine
In 1809 Nicolas Vauquelin (1763-1829) discovered nicotine in the leaves of two varieties of the tobacco plant, Nicotiana tabacum and Nicotiana angustifolia (Vauquelin, 1809). The active component was separated by bruising the leaves, mixed with a little water, in a marble mortar, followed by expression between cotton cloths. The accompanying green matter was separated by filtration through a bloating paper. After a detailed chemical treatment, the juice was found to contain an animal matter (comprising nitrogen), calcium malate with an excess of the acid, potassium nitrate and chloride, and a peculiar acrid, volatile, and colorless principle, soluble in water and alcohol that Vauquelin claimed was different from all other known principles of the vegetable kingdom. This was the principle that imparted prepared tobacco its peculiar character. Vauquelin did not provide information about the composition of the principle. In 1828 Christian Wilhelm Posselt (1806-1877) and Karl Ludwig Reimann (1804-1872) determined some additional properties of nicotine, including its being poisonous but did no determine its composition (Posselt & Reimann, 1828). V. Ortigosa repeated the work of Posselt and Reimann and claimed that these scientists had isolated an impure product; it contained water and alcohol. Ortigosa tried unsuccessfully to eliminate the water by means of fused KOH. Unfortunately, the alkali removed partly the nicotine and left a product having different properties, particularly the smell. Nevertheless, he was able to deduce the formula of nicotine from the composition of its double chlorides with mercury and with platinum. This indicated that nicotine contained, by weight, 73.26% carbon, 9.65% hydrogen, and 17.09% nitrogen, corresponding to the formula C10H16N2 (Ortigosa, 1842).
In 1842 Barral published a short note about nicotine, which he called tobacco alkali, reporting his determination of the composition of the principle (Barral, 1842, 1843). He treated 20 kg of dry and crushed leaves of tobacco of Alsace with water acidulated with sulfuric acid and after three days removed the juice by pressing. He then evaporated the liquid to half its initial volume and steam distilled the residue over calcium sulfate. The nicotine carried over by the water was separated by means of ether and the solvent eliminated by slowly heating at increasing temperature up to 140 oC. The new residue was distilled again over lime using a stream of hydrogen as carrier. The purified nicotine (16 g) was treated with a current of chlorine and found not to contain ammonia. Barral described it as colorless, transparent, and anhydrous liquid that in contact with air turned brown and thicker. The liquid did not solidify even when cooled down to -10 oC and volatilized at 250 oC leaving a carbonaceous residue. According to Barral, nicotine was an extremely violent poison; 5 mg of it put in the tongue of a big dog caused its death in less than 5 minutes. Nicotine turned blue wet litmus paper and behaved like a fixed alkali. It combined with acids with release of heat; the halogen salts were deliquescent and crystallized with difficulty. All the salts were soluble in ether. Combustion analysis indicated that it contained, by weight, 73.33% carbon, 9.42% hydrogen, and 17.04% nitrogen, corresponding to the formula C20H16N2 (with C = 6). Today we know that nicotine contains, by weight, 74.03% carbon, 8.70% hydrogen, and 17.17% nitrogen, corresponding to the formula C10H14N2. Barral described in detail the preparation, composition, and properties of nicotine hydrochloride, chloroplatinate, and the double chlorides with mercury, tin, and iron chlorides. He wrote that all his results indicated that nicotine was a well-defined alkali (Barral, 1842, 1843).
In a following paper Barral mentioned that all the publications regarding tobacco reported inconsistent results because they were not carried on the same variety of the plant and not on leaves prepared under the same circumstances (i.e., fresh, dry, non-treated, elaborated, smoked, taken from the same part of the plan, etc.) (Barral, 1845). For these reasons, Barral decided to re-study the analysis of tobacco, using several parts of the plant (ashes of the leaves, ribs, roots, stalks, seeds, etc.) of French and foreign origin. His results indicated that the amount of ashes present increased in the order grains, stalks, ribs, and leaves. In round numbers, the weight of ashes was 4% in the grains 7% in the roots, 10% in the stalks, and 23% in the leaves. The different classes of tobacco examined came necessarily from different terrains, which led to a large variation in the composition of the cinders. Nevertheless, a principle enunciated by Liebig seemed to be true: a base replaced another, if it was equivalent. Except for the roots, the quantity of oxygen found in the ashes of the leaves, stalks, and fibers, averaged 13%. The roots contained a very large amount of silicon, at least eight times larger than other parts of the plant. The amount of calcium increased in the order roots, stalks, ribs, and leaves, while the amount of potassium decreased in the order stalks, ribs, and leaves. The quantity of nitrogen was very large, the average in the leaf being 5 to 6%. The grains contained about 5% of nitrogen and about 10% of colorless fatty oil. Barral mentioned that the small size of the grains had led many researchers to neglect them; a cubic centimeter of them contained about 11,000 units, and weighed 0.5118 g. The juice obtained by macerating the leaf in water was strongly acidulated. Vauquelin attributed this acidity to the presence of malic acid; but Barral, having crystallized it as micaceous strips, proved that the acid was one peculiar to the plant, and he named it nicotic acid. Nicotic acid was soluble in water; it produced an insoluble salt with lead and crystallizable salts with nicotine, ammonium, and KOH, and had a composition equivalent to the formula C6H2O3 + H2O. The large tendency to form double salts suggested that the actual formula was two times larger. Nicotic acid was decomposed by heat and sulfuric acid into acetic acid and carbon dioxide. According to Barral nicotic acid was related to metacetonic acid (propionic acid) the same as oxalic acid was to acetic. Tobacco also contained a nitrogenated essence, nicotianine that contained, by weight, 71.52% carbon, 8.23% hydrogen, 7.12% nitrogen, and 13.13% oxygen (Barral, 1845).
In 1843 William Christopher Zeise (1799-1847), analyzed the smoke of tobacco and reported that the principal constituents were an empyreumatic oil, CO2, ammonia, butyric acid, a hydrocarbon, and resinous compounds. In his apparatus the smoke of a pipe was aspired through a series of washing bottles containing different reagents. Zeise mentioned that the butyrates extracted from tobacco, when not too diluted, gave a green precipitate with cupric chloride and a white one with basic lead acetate, mercurous oxide, or silver nitrate. Curiously, he did not report the presence of nicotine, although he reported that he had detected nitrogen in quantities below 3% (Zeise, 1843). Louis Henri Frédéric Melsens (1814-1886), who had been working on the question of the analysis of tobacco smoke for several years, was surprised that Zeise had missed the presence of nicotine, a component present in substantial amounts (Melsens, 1843). He now decided to experiment on tobacco leaves from Virginia, known to produce extremely strong cigarettes. He used an experimental setup very similar to that of Zeise and noted that the smoke that condensed in the first flask produced brown alkaline water, having a nauseous odor, effervescing with acids, releasing ammonia in contact with bases, and extremely poisonous; half a spoon was enough to kill a dog instantly. Melsens used the procedure suggested by Barral to separate the nicotine contained in the first vessel. After purification, he was able to obtain about 30 g of a colorless liquid having all the physical and chemical properties assigned by Barral to nicotine. Further purification and elementary analysis indicated that nicotine contained, by weight, 74.3% carbon, 8.8% hydrogen, and 17.3% nitrogen, corresponding to the formula C20H14N2 (This differs from the correct formula C10H14N2 because of the erroneous value of the value of the atomic masses used then). Melsens wrote that his formula differed from the one proposed by Barral by one equivalent less of hydrogen (Melsens, 1843). In 1846 Jean Jacques Théophile Schloesing (1824-1919) published the results of his experiments about nicotine and claimed that the correct formula of nicotine was that proposed by Melsens and not by Barral (Schloesing, 1846). This claim was promptly criticized by Barral who showed that the percentage composition of nicotine given by him in 1842 with those reported by Melsens and by Schloesing were significantly similar and that his error in calculating the corresponding formula was due to the values of the atomic masses then available, and to the fact that the composition of water at his time (HO) was in error (Barral, 1847a).
Chemical balance of the body
Barral wrote extensively about the question of chemical balance of the body of living animals (Barral, 1848, 1849a b c, 1850a b, 1863). He defined the problem as follows: "Knowing the amount and elemental composition of the solid and liquid foods ingested daily and the amount and elemental composition of the diverse evacuations, transpirations and excretions, establish the gain and loss equations of the human body (Barral, 1848, 1849a)." According to Barral, there were three research study subjects that might throw light on the maintenance of life in humans and animal: (1) the foods ingested, (2) the gaseous liquid and solid products of the process, and (3) the phenomena taking place during food assimilation and the continuous change of tissues. The first reports of these subjects had lacked precision and had been obscured by preconceived ideas with empiricism taking the place of well-established facts. The advent of pneumatic physics had substantially improved knowledge, it was now accepted that air introduced something in the lungs and that only part of the air was appropriate for this purpose (Barral, 1849a). Robert Boyle (1627-1691) had conducted numerous experiments trying to isolate the portion of the air that was breathable (Boyle, 1660). Joseph Priestley (1733-1804), after discovering oxygen had written that "it was appropriate to see that it was through this gas, dephlogisticated air, that atmospheric air allowed the maintenance of life", although he thought that the respiration phenomenon in animals had simply phlogisticated the air in the same manner as it did in the calcination of metals, fermentation, and putrefaction (Priestley, 1774-1777). Antoine-Laurent Lavoisier (1743-1794) and Armand Seguin (1767-1835) had discovered that "the inspired air was replaced by CO2 in the expired fluid and that the conservation of animal heat was mostly due to the heat released by the combination of pure air, respired by animals, with the base of fixed air proportioned by blood": oxygen of the air combined with the carbon in the blood, releasing CO2 and heat (Seguin & Lavoisier 1789). Afterwards, Lavoisier and Seguin found that the volume of CO2 generated was less than that of the corresponding amount of oxygen meaning that part of this gas combined with the hydrogen of blood to generate water (Lavoisier & Seguin, 1789). This water joined that of foods to be exhaled by air or by cutaneous transpiration (Barral, 1849a).
Barral wrote that the production of CO2 during respiration was afterwards verified by many scientists, among them, Davy, Humboldt, Spallanzani, Dumas, Regnault, Andral and Gavarret, etc. (Barral, 1849a). These scientists examined the products of respiration, the ratio of water and CO2 exhaled, and their relation to the amount of heat released. The nitrogen released and the water lost by respiration and by skin transpiration did not escape the attention of their attention and they tried to connect it with the water and urea in urines. Jean-Baptiste Boussingault (1802-1887) and Justus von Liebig (1803-1883) had made some preliminary examination of the elemental composition of the food ingested by animals and by humans, and their evacuations, without reaching significant results (Boussingault, 1839; Liebig 1842).
In a first long paper on the subject (42 pages) Barral described the five experiments he had conducted on himself (2), a small boy (his son, 6 years old), an old man employed in Barral's laboratory, and a 32-year-old woman, during 5 days, in winter and in summer, a lapse of time he believed would yield the same results as experiments performed for a longer period of time (Barral, 1849a). The variables measured were the water, organic matter, chlorine, and fixed mineral salts present in foods and evacuations. He remarked that he did not analyze the lung and skin transpiration. The organic mater was analyzed for carbon, hydrogen, nitrogen, and oxygen. The results were presented in two types of tables, one describing the total amounts of ingested and evacuated matters, the other, the pertinent analyses. Particular interest was put on the role played by salt (NaCl) in human economy. This was achieved by a careful analysis of the chlorine content in the different materials. The first table reported the date, average temperature, average atmospheric pressure, the weight of solid and liquid foods, of the food bowl, of urine, excrements, and of oral and nasal secretions, for everyone tested. The following table detailed all the foodstuffs (bread, meat, potatoes, carrots, butter, water, sugar, etc.) eaten during the five days, their individual weight and percentage of the total, the amount of organic matter dried at 110 oC, and the content of chlorine and fixed salts. The third table gave the analytical data of the evacuations (urine and excrements) as water, dry organic matter, chlorine, fixed salts, and weight. The next three tables gave the elementary analysis of all the above substances (Barral, 1849a).
For burned carbon, Barral found the same proportions that Gabriel Andral (1797-1876) and Jules Gavarret (1809-1890) had determined by a different procedure (direct analysis of the products of respiration) (Andral & Gavarret, 1843), with the additional fact that the amount of carbon consumed in winter was about 20% larger than that consumed in summer (Barral, 1848, 1849a). In addition, he found that the quantity of nitrogen present in the food was higher than that in the evacuations, so that a part of this gas had to be exhaled by perspiration. This portion constituted about 1/3 to 1/4 of the nitrogen consumed but only 1% of the CO2 produced. In a balanced diet, the ratio carbon/nitrogen was about 100 to 8. Hydrogen and oxygen were not in the ratio present in water; the feed contained an excess of hydrogen; hence they were partially burned by the oxygen of respiration. This hydrogen was, approximately, equal to 1/3 of the carbon transformed into CO2; also, it was not all the hydrogen contained in the food. The evacuations were richer in hydrogen than food, in the ratio 1/5. The oxygen necessary to burn the carbon and hydrogen of the foods into water and CO2 during respiration was present in the food bowl in the ratio one to three. Barral found that the amount of water passing through the human body was considerable and undoubtedly played an important role in the functions of life. Water, natural and formed during respiration and digestion, constituted about 67% of the food bowl, increased by the atmospheric oxygen combining with it. The quantity of water contained in the respiration was slightly larger than that in the evacuations. In an older person, the transpired water was reduced to about one third that present in the urine and excrements.
Barral found that the amount of mineral salts present in the food bowl was larger than the one present in the evacuations, a result that could only be explained by problems in the experimental procedures. The amount of salt was determined by incineration; for certain substances (i.e., coffee, dry broth, and meat) it was very difficult to obtain perfectly white cinders (hence, exempt of carbon). In addition, the cinders of food normally effervesced in contact with acids, and the CO2 was usually accounted in the weight of the cinder (Barral, 1849a).
According to Barral, the chemical balance of the human body could be represented (%) by the following equation (Barral, 1848, 1849a):
Solid and liquid food (74.4) + oxygen (25.6) = perspiration water (34.8) + carbon dioxide (30.2) + evacuations (34.5) + other losses (0.5)
An important question was the amount of heat necessary for maintaining constant the temperature of the body. Knowing the amount of water leaving the lungs as steam and the skin as perspiration allowed an easy calculation of the amount of heat required for its evaporation. Similarly, it was easy to calculate the amount of heat (enthalpy) carried by the air leaving the lungs, assuming it was at body temperature (37 oC) and saturated and knowing the specific heat of the gas (0.267). The corresponding total heat balance indicated that the heat lost per day by radiation and contact was 30,000 in summer and 42,000 in winter (Barral did not indicate the units; he only indicated that the pertinent figure was calculated assuming that the caloric power of carbon and hydrogen were 7,200 and 34,600, respectively), and was represented (%) by the following equation (Barral, 1848, 1849a):
From water perspired (24.1) + respiration air (7.3) + taken by the food bowl (2.2) + evacuations (1.8) + radiation and contact (64.6) = 100.
The next publication was related to the chemical balance of the sheep (Barral, 1849c). Barral wrote that the general results agreed with those found for the human body and with respect to perspiration, they agreed very well with those reported by Victor Regnault (1810-1878), Jules Reiset (1818-1896), and Eugène Auguste Nicolas Millon (1812-1867) (Regnault, Reiset, & Millon, 1848). Nevertheless, certain differences merited the attention of sheep growers since the balance indicated this animal happened to be fed with substances not so appropriate for their assimilation, which were discarded in the excrements. Barral remarked that his experiments were addressed to find the effects of a salty diet. In his experiments the sheep tested, commonly fed with salt, had been subjected to a five-day long test during which all the food and the evacuations were dosed and analyzed. Each day it received 12 grams of salt. After this period, it had been tested for another ten days during which no salt was provided, and all the food and evacuation dosed and analyzed. These cycles were repeated several times, under more or less the same conditions. The analyses of the urines had indicated a remarkable result: the sodium chloride led to a strong increase in the amount of nitrogen evacuated by this route. The salty regime led also to an increase in the proportion of uric acid. This increase of the proportion of nitrogen, accompanied by a decrease in the amount of nitrogen in the gas released to the atmosphere, was completely in accordance with variations already observed in the nature of the solid matter present in the urines, when the diet was changed (Barral, 1849c).
Barral wrote that Jean-Baptiste Dumas (1800-1884) and Jean Baptiste Boussingault (1802-1887), in their book about the chemical balance in organized beings (Dumas & Boussingault, 1841), had presented the general characteristics of a significant natural law but had left many questions unsolved, among them, the recycle of nitrogen of animals to the atmosphere and from them, back into plants and animals (Barral, 1863). Some experiments on animals were direct, they examined the expired materials; others were indirect, they determined the amount of nitrogen by difference between the nitrogen ingested and the amount of nitrogen excreted. The results of several scientists, among them Dulong, Despretz, and Regnault and Reiset, were very conclusive: animals expired an amount of nitrogen higher than that inspired by respiration. This difference was not very large, about 1% of the CO2 released to the atmosphere; this amount varied according to the diet and health state of the animal. This way of considering the facts was enough for physiological purposes but not for the Earth physics and for rural economy. A correct determination of the ratio between nitrogen ingested by animals and the amount not restituted by secretions was of fundamental importance for agriculture. Barral had already made public the results of his experiments on human beings (Barral, 1849a). They indicated that an adult man exhaled 9 to 14 g of nitrogen per day, a five-year old child 3 g, and an adult female, about 12 g/day. These amounts corresponded to a little more than one third of the nitrogen contained in the food intake. In another work he had reported that a sheep inhaled about 6 g/day of nitrogen, or about one-quarter of the amount present in the diet. These numbers indicated that a beast at the height of his meat, milk, or work, consumed per year, an amount of food corresponding to about 6,000 kg of hay. This result confirmed the claim of Boussingault that domestic animals were not producers of fertilizer; they were actually consumers of fertilizer. They converted the organic matter they consumed into foodstuff assimilable by plants at a very expensive prize. In other words, the fertility of a rural property based on beasts could only be maintained by the import of external fertilizers, purchased, or present in the irrigation. In other words, it implied the possible non-stability of the composition of the atmosphere. Otherwise, it was necessary that natural causes restituted to the atmosphere about 4 to 5 kg of nitrogen per hectare per year (assuming the presence of 100 kg of live beings per hectare) (Barral, 1863).
Barral wrote that all the efforts to prove the direct assimilation of gaseous nitrogen by plants had failed (Barral, 1863). Nevertheless, Boussingault experiences proved that plants imported part of the nitrogen found in their tissues from a source different than the soil, either from the fertilizers in contact with their roots or from atmospheric nitrogen absorbed indirectly from the atmosphere. Barral mentioned that in order to solve this problem he had conducted between 1851 and 1854 experiments with the rainwater of Paris and surroundings. These had shown that ammonium nitrate was present in the atmosphere and that the rain entrained it (Barral, 1852b). Nitric acid and ammonia did not exist accidentally in the atmosphere; but the two compounds were found normally, and in a detectable amount, in all the rainwaters collected. Nevertheless, their amount was not enough to justify completely the deficit. Barral believed that the nitrification of the atmospheric nitrogen present in the soil was probably the source of the missing nitrogen. He had looked for the presence of nitrates in drainage waters and found that these waters were richer in nitrogen than the water originating from the most fertile soils. This assumption was supported by the common practice of deeply working the soil to facilitate its aeration (Barral, 1863).
The role of sodium chloride
According to Barral, chemical analysis had indicated the constant presence of sodium chloride or sodium in every part of the animal body, in their secretions, and in their food. Was this fact an absolute proof of the constitutional need of this salt or its elements, or were animals organized in such way as to be able to live without salt, sodium, and chlorine? Could it be that salt had been introduced as an accessory and worthless product in the food, and hence in the organism so that it was a deviation product of the civilization state (Barral, 1849b) Barral went on to analyze the possible ways of answering the question. It was well known that there were many liquids in animal economy (i.e., bile, blood, sperm, etc.) that could not exist without the presence of an alkaline base. The question then was if sodium could be replaced by a mineral or organic alkali, in the same it took place in many vegetables. According to experience, this was hardly possible. In addition, it was not possible to test this possibility on human beings. Perhaps there was a wild tribe somewhere where the use of salt was still unknown, or maybe its use had been suppressed or restricted by certain reasons. Historical facts indicated that humans had used salt since ancient times for religious or profane uses. Use of salt for seasoning was already recorded in the writings of Homer. In the book of Job in the Bible it was written: "Is tasteless food eaten without salt, or is there, flavor in the sap of the mallow? (Chapter 6, verse 6). Barral went on quoting many sources to prove that salt was widely used in all cultures. There were also several reports on the dangerous consequences of using diet without salt. The avidity of salt by animals was a fact observed universally; some claimed that observation of this custom had led humans to copy it (Barral, 1849b).
Another difficult question to answer was the amount of salt that had to be present in the food bowl to produce its maximum useful effect (Barral, 1849b). Many experiences had been made for this purpose; they were usually based on comparing two groups of animals, their weight ratio against the production accessories such as milk and wool, their increase in weight, mortality, etc. Only two researchers had studied the effect of salt on the maturing of cows and sheep. Boussingault had found that intervention of salt had a favorable effect on the maturing process of cows, and thus on the economics of growing this kind of animals (Boussingault, 1839). Frédéric Lequin, a grower of sheep and lamb at Neufchâtaux (Vosges), had obtained similar results. The effect of amount of salt on the fattening of sheep and lamb fed the same diet, were contradictory, some claimed that increasing the amount of salt led to higher weights, other claimed the opposite result. Barral wrote that M. de Béhague, a grower of cows, had reported that an increase in salt had led to an increase in the amount of food ingested and a decrease in the weight of the cow. Salt had provoked an increase in appetite, without helping in their development and without increasing their capacity of assimilation (Barral, 1849c, 1850a).
Barral studied the effects of a variable amount salt on the evacuation of excrements and urines in humans, horses, bovine, and ovine (Barral, 1849c). His results indicated that the amount of salt contained in the excrements was a very small fraction of the total salt consumed so that most of the salt was absorbed in the circulatory system. This result indicated that the actual effect of the salt reflected in the changes of the tissues and not in the assimilation act of food by the digestive system. In every animal species there was a clear constancy of the ratio of the dry matter contained in the excrements and that in the food bowl (0.043 in humans, and 0.376 and 0.466 in bovine and ovine, respectively (Barral, 1849c).
The above results that salt resulted in an increase in the amount of water consumed, and hence, salt was almost completed secreted by the kidneys and appeared dissolved in the urine. Determination of the true ratio between the volume of the urine and the amount of water in the food bowl was complicated by external factors such as temperature and humidity. A clear result was that independently of the mass of the bowl (larger or smaller), sodium chlorine had a clear effect on the amount of solid organic matter present in the urine; it facilitated the growth of tissues and the evacuation of matter inadequate for the maintenance of life. A saline regime increased the amount of nitrogenous matter evacuated by the urine (in the form or urea and uric acid) (Barral, 1849c). Barral also studied the effect of dietary salt upon the maintenance of molecular forces. He found that the most important effect of salt was to increase the total of mechanical effects, or in simple words, the amount of daily work that animals could produce (Barral, 1849c).
Barral published a long book (500 pages) detailing his work on the chemical balance of the body of a living being, particularly on the question on the role of sodium chloride (Barral, 1850b).
Influence of rainwater on vegetation
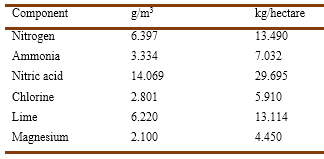
Barral published several papers on the composition of rainwater and its influence on vegetation (Barral, 1852a b, 1860, 1861, 1882b). He mentioned that Jovita Malaguti (1802-1878) had reported that drying rainwater left a rather large residue composed of the powders that were always present in air, plus certain soluble salts such as sodium chloride and ammonium nitrate. The water vapor contained in the atmosphere, falling as rain, would entrain these chemicals, which would then penetrate the soil and exert a very favorable influence upon vegetation. Clearly, these solutes constituted one of the elements supporting the existence of vegetation (Malaguti, 1848, Barral, 1852a). Boussingault had written that air contained minute amounts of ammonia vapors; that nitrogen was able to penetrate directly the plants organism so that if the green parts were able to fix it, then it would be carried by the aerial water aspired by the roots (Boussingault, 1838). Barral wrote that the readings of the gauges of the Observatory of Paris showed that the rains fallen between July and December 1851 had the following metrics (Barral, 1852b):
A similar table was presented for the first semester of 1852. According to Barral, the statistical data for the year comprised between July 1, 1851, and June 30, 1852, indicated that the amount of rain fallen contained 22.5 kg of combined nitrogen per hectare, corresponding to 12.5 kg in the form of nitric acid, and 10 kg in the state of ammonia.
Deposition of metallic gold
In 1846 Barral published two papers about the conditions required to deposit metallic gold over different metals, as a continuous and adherent layer, using the Elkington bath (a solution of potassium cyanide), and the same simple immersion used in gilding by the wet procedure (Barral, 1846). This procedure was not clearly understood, and Barral tried to find an explanation for it. In the first part of his paper Barral gave a short summary of the state of the art. Gilding was based on the general principle of the precipitation of metals from their solutions by other more oxidizable metals, as the precipitation of copper by iron from a solution of cupric sulfate and the dissolution of iron in an equivalent proportion to the copper deposited. It was accepted that in order to obtain a better precipitation it was more convenient to use a solution slightly acidulated. The free acid acted upon the active metal and determined a current that stimulated the decomposition. In the case of gilding this modification presented some problems when trying to cover with gold the parts etched or chiseled with care; the free acid could deteriorate the object submerged in the solution, and alter, for example, the delicate parts of a clock. Antoine Baumé improved the process employed by watchmakers by dissolving the gold in aqua regia, evaporating and crystallizing the solution, and using an aqueous solution of the crystals to gild well-polished copper or iron surfaces, followed by water washes. This resulted in a beautiful and brilliant gild not presenting the black points accompanying the standard gilding method (Baumé, 1773). In spite of this, the gilding process was slow and tricky, as shown by the possible reactions Au2Cl2 +3Cu = 3CuCl + Au2 admitting the formation copper dichloride, or Au2Cl3 + 6Cu = 3Cu2Cl + Au2, admitting the formation of copper protochloride. In both cases, a large amount of copper added to delicate objects and deteriorated them. The basic problem remained: how to neutralize part of the acid of gold(III) chloride. It was not until 1840 that an elegant solution was found to the problem (Barral, 1846).
In 1836, 1837, and 1840 patents were assigned in England and France to George Richards Elkington (1801-1865), Henry Elkington (1810-1852), and John Wright (1808-1844) for a gilding process involving a bath with potassium bicarbonate (Elkington, 1836, 1837; Wright, 1840). Barral prepared the bath according to Baumé and the patents: the gold was dissolved in aqua regia and evaporated in the presence of potassium bicarbonate to eliminate most of the acid. The jewelry was added when the solution achieved a green color. Gilding occurred rapidly, part of the copper of the jewelry dissolved to replace the deposited gold, and the solution acquired a blue tint. A black precipitate formed during all these effects, which deposited at the bottom of the bath. Wright and Elkington assumed that as a result of boiling a solution of gold trichloride in the presence of potassium bicarbonate and organic substances, gold was reduced to its lower chlorinated state. Dumas had accepted this explanation and admitted that in the gilding of brass by the wet method the chlorine of gold (1) chloride took hold of one equivalent of copper and precipitated one equivalent of gold (Dumas, 1841). Louis Figuier (1819-1894) had contested this explanation and claimed that gold(I) oxide precipitated as it was formed, forming the black precipitate that was always found at the bottom of active baths. An oxide, more oxygenated than auric acid and extremely unstable, was formed, which was appropriate for precipitating gold (Figuier, 1844). Hence, two opposing explanations were possible: reduction of gold and gilding by the salt at its minimum oxidation state, or super oxidation of gold and gilding by the higher oxide (Barral, 1846).
Barral prepared a bath containing a known amount of gold, gilded a certain quantity of jewelry, and analyzed the bath and the black precipitate. His results indicated that the copper present in the bath and in the precipitate was equivalent to Cu2, and in the precipitate, to Au2. This meant that that the gilding agent was gold(I) chloride, according to Au2Cl + 2Cu = Cu2Cl + Au2, or that an unknown gold chloride, intermediate between gold(I) chloride and gold(III) chloride reacted according to Au2Cl2 + 2Cu = 2CuCl + Au2. In the black precipitate Barral found calcium carbonate, copper oxide, and Cassius purple (a red pigment formed by the reaction of gold salts with tin(II) chloride)
Analysis of the bath after the gilding confirmed Figuier's claim that gold was present in its highest state of oxidation, that the gilding process was independent of the organic matter, and that the only purpose of potassium bicarbonate was absorption of the excess of chlorine. These results led Barral to use the bath for an indefinite amount of time, instead of only once. Gold(III) was added as the bath became exhausted and the potassium became potassium chloride and chlorate. Addition of potassium bicarbonate increased the effectiveness of the bath, as explained by the reaction 6Au2Cl3 + 6KO + 12Cu = 12CuCl + 5KCl + ClO4KO + 6Au2. In a brilliant stroke, Barral deduced that since gilding by immersion was simply a chemical reaction based on Bergmann's law for metallic precipitations, and all chemical actions generated electricity, then it would be possible to gild by immersion practically all metals, to any desired thickness, by taking advantage of the liberated electricity. This assumption was verified by actual experiences carried by professional gilders, and by gilding done with an electrochemical process using only one pair, without using diaphragms (Barral, 1846).
Barral also verified the results obtained by Antoine Cesar Becquerel (1788-1878) about the influence of polishing the surface to be gilded by the moist process: a copper surface polished with the most care takes the least gold (Becquerel, 1843). Barral found that a badly polished surface, but well-tempered and cleansed, could be coated by immersion with a layer of gold, the thickness of which was solely limited by the quantity of gold contained in the solution. In this manner, Barral wrote that he had succeeded in gilding to any thickness, with the most beautiful tints and colors, copper, silver, platina, iron, and lastly gold itself, with Elkington's bath, by the simple immersion of the metals in the presence of copper, zinc or lead. Barral provided a table describing the behavior of a single metal (platinum, silver, iron, tin, copper, zinc, and lead) or a mixture of two of these (i.e., platinum + zinc, silver + iron, silver + lead, etc.), within the alkaline gold bath (Barral, 1846).
Electromagnetism
In 1820 François Arago (1785-1853) demonstrated that iron filings became magnetized under the influence of an electric current (Arago, 1820) and in 1827 André-Marie Ampère (1775-1831) and other scientists suggested using this effect to build artificial magnets, permanent or temporary (Ampère, 1827). According to Barral, magnets had become a common tool in every laboratory; they consisted ordinarily of bars of soft iron curved in the form of a horseshoe, the arms of which were wound round with isolated copper wire through which was passed an electrical current. The magnetic effect lasted if the current was flowing. The resulting magnetized soft iron could support a considerable weight; Claude Pouillet (1790-1868) had manufactured the most powerful electromagnet, able to lift about 410 kg) (Pouillet, 1832). In addition, Michael Faraday had experimented on the action of magnetism upon transparent bodies traversed by polarized light (Faraday, 1846).
According to Barral, despite the abundant information about this inductive phenomenon, the relation between the energy of an electric current, the number of metallic turns wrapped around the soft iron, the magnetism actually produced, the weight lifted, etc. was far from being clear. For these reasons, he decided to conduct additional experiments about the maximum weight that an electromagnet could lift (Barral, 1847c). In order to obtain a constant current Barral used a Bunsen battery connected to a liquid rheostat (formed by two plates of platina), dipping into water more or less acidulated, and a galvanoscope needle. Throughout all experiments he took the precaution always to bring back the needle to the same deviation. His first objective was to find the influence exercised by the weight of the armature or contact, which completed the magnetic circuit of soft iron and served to attach the platform bearing the weights. He found that increasing the weight of the armature increased the total supported weight in an equal proportion, until it reached a maximum value when the weight of the armature equaled that of the electromagnet. At constant current and constant number of spiral turns of wire, the sum of the weights increased also when augmenting the weight of the induced iron (Barral 1847c).
Barral found also that the direction in which the traction exercised by the armature took place had a strong influence on the effort required to separate the armature from the magnet. This effort diminished as the iron bars of the electromagnet were removed to a distance from the direction of their axis. The minimum value occurred when the power of traction operated in a perpendicular direction to the axis. Barral provided a table giving for seven weights of the armature (increasing from 0.20 to 15.00 kg), the weight carried vertically, the traction exercised horizontally and at two angles from the vertical (41°9'10" and 63°12'). The best results were obtained with an armature weighing 9.25 kg; it supported vertically a weight of 295 kg, and 131 kg horizontally. Barral also investigated the effect of friction exerted horizontally to separate the armature. He found the mean of the coefficients of friction was 0.230, while the mean relation of the tractions, horizontal and vertical, during magnetic action was 0.357, indicating that the excess of 0.127 arose from magnetic action. Application of the formula of the laws of friction to oblique tractions led to the same result: the magnetic action causing the iron to adhere to the magnet did not resolve itself into a normal action, as regards the surfaces in contact; there was also a molecular action, variable according to the different inclinations and surfaces (Barral, 1847c).
Barral carried many experiments to determine the influence of the distance (x mm) between the armature and the electromagnet upon the weight carried (y kg). His results indicated that this effect could be represented by the formula y = A/(B + C x), where A, B, and C, were functions of the strength of the current and the weight and shape of the electromagnets and their armatures. For the conditions employed, A was larger than 1, B was a fraction, and C was comprised between 1 and 2. These values showed that the weight carried decreased very rapidly with the distance (Barral, 1847c).