Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Ciencias Forestales
versión On-line ISSN 2310-3469
Rev CFORES vol.12 no.1 Pinar del Río ene.-abr. 2024 Epub 01-Abr-2024
Original article
Morphological assessment of pollen on the honey tree species from the tropical dry forest of the Quimis enclosure
1Universidad Estatal del Sur de Manabí, Manabí. Ecuador.
With the objective of evaluating the morphology of pollen in honey species from the tropical dry forest of the Quimis enclosure, five apiaries distributed in said plant formation were selected, the pollen loads obtained directly from the apiaries were collected and the bee flora was recorded according to the flowering time. The results revealed 21 honey species belonging to 13 families and 21 genera, being Convolvulus arvensis, Eriotheca ruizii, Ceiba trischistandra and Prosopis pallida the most common species and the most abundant family was Fabaceae. In relation to the pollen units, 0.95% corresponds to the monad type and referring to symmetry, 0.67% is characterized by being of the radiosymmetric type, while 0.48% has tricolporate openings. Regarding the shape of the exine, 0.43% exhibited a psyllate shape, and 0.38% showed a spheroidal shape. The months of greatest flowering coincided with June, July and August, which correspond to the dry season. The vast majority of nectariferous and polliniferous species were dominant, with the exception of Bonellia sprucei, Caesalpinia paipai and Trema micrantha, Muntingia calabura, Cojoba arborea, Sarcomphalus thyrsiflorus, Bursera graveolens, which only provide pollen and Cordia lutea and Cynophalla sclerophylla, which are nectar producers.
Key words: beekeeping; apiaries; honeycomb; pollen morphology.
INTRODUCTION
In natural forests and other forest areas within them, the flora used by bees is found, where bee pollen can be considered as a by-product of the forests and promoted as a non-timber forest product (NTFP), in such a way that, allows beekeeping to be considered as an activity that can be integrated into the management and conservation of forest species and the ecosystems where they live (Chamorro et al., 2013).
Beekeeping is an activity that takes advantage of vegetation, both in its natural and altered state, as well as agricultural and forestry crops. In this same sense, there are plant species that flower very early, and encourage the hive to begin reproducing and other species. which they do successively throughout the year (Paredes, 2017).
Studies carried out by the (Food and Agriculture Organization of the United Nations [FAO], 2018) reveals that pollination is the most important process in nature that contributes to biodiversity. Because 90% of flowering plants depend on pollination to survive, for this reason, bees maintain forest ecosystems, conserving their biodiversity.
On the other hand, Méndez et al. (2021) indicated that the relationship between bees and floral resources is carried out through palynological studies, which allow the identification of the different botanical species that contribute to the food of bees.
In the province of Manabí, currently various cantons are dedicated to beekeeping, emphasizing the production of honey, propolis, wax, among others. The Quimis area of the Jipijapa canton is considered one of the areas populated by many trees, but especially Ceiba trischistandra. (A. Gray) Bakh. (ceibo) and Prosopis juliflora var. juliflora (algarrobo), which are direct sources of nectar and pollen, which is a place inhabited by beekeepers who learned to generate economic income through this activity and everything it encompasses. For all the above, the objective of this study was to assess the morphology of pollen of honey species from the tropical dry forest of the Quimis enclosure, oriented towards conservation.
MATERIALS AND METHODS
Characterization of the study area
This research was developed in the active apiaries in the area of influence of the Quimis enclosure, which is located in the Membrillal parish, in the Jipijapa canton. This facility is located at kilometer 21 of the road that connects the cantons of Portoviejo and Manta, in the province of Manabí, in the region of the Ecuadorian Pacific coast.
Methodology
Identification of the botanical and morphological origin of bee pollen from apiaries in the Quimis enclosure
Apis mellifera hives were selected, in five active apiaries within the enclosure, whose distance from the house to the apiary varies between 300 to 500 m, following the methodology of De Boada et al. (1987) y De Boada and Cogua (1989).
To collect pollen, pollen traps were placed in the entrances of the hives (entrance of the hive), for a period of 12 to 24 hours specifically on three business days with permission from the beekeeper for two weeks, in the months of May, June and July 2023, according to the methodology proposed by Hidalgo et al. (1990) y Sayas and Huamán (2009).
To bidentify the pollen, the samples were transferred to the biotechnology laboratory of the State University of the South of Manabí (UNESUM) and observed with an optical microscope model Better Scientific with objective lens of 4/0.10.
The pollen samples were placed in Petri dishes, after having been sieved to eliminate impurities. They were then subdivided by apiary and, within each apiary, additional subdivisions were made according to the color and weight of the samples.
For the preparation of the samples, two solutions were made. The first combined glycerin and distilled water (5 ml of each) in Petri dishes (Insuasty et al., 2017), mixing them until obtaining a homogeneous consistency. Clean pollen grains were added to this solution for hydration and uniform distribution, allowing them to rest for 48 hours.
After this period, a drop of the solution was placed on a slide, covering it with a coverslip. For the second preparation, two drops of fuchsin solution diluted in 1 ml of ethanol were used, in order to highlight the characteristics of the pollen. This process was repeated three times to ensure the reliability of the analyses.
For a better visualization of the pollen morphology (Figure 1 to 4), the samples were examined under a microscope with a Euromex model camera with a objective lens of 40/0.65; in the parasitology laboratory of the UNESUM Clinical Laboratory program.
Finally, to determine the botanical origin of the pollen, virtual Palinotheques and resources such as the quick guide to Pollen of the Galapagos Islands (Jaramillo and Del Mar, 2011), the Photographic Catalog of species of Bee Flora (Velandia et al., 2012) and Montoya et al. (2014) were consulted.
Once the species were identified, the morphology of the pollen was recorded, for which the characteristics described by Jaramillo and Del Mar (2011) were taken into account, namely:
Symmetry (radiosymmetric, bisymmetric, asymmetric)
Size (< 10 µm very small pollen grains and > 200 µm giant pollen grains)
Pollen grain units (monad, dyads, tetrads, polyads)
Exine form (psyllate, fossulate, foveolate, scabrid, equinate, baculate, gemate, pilate, reticulate, warty)
NPC system through the number, position, pollen characters.
Form that pollen presents.
According to the aforementioned authors, microscopic analysis of pollen samples collected in a specific period reveals the varieties of species that bees have used as pollen sources.
Parameter Evaluation
To evaluate the population parameters, office tools such as Microsoft Excel were used, as well as specialized programs such as Infostat and GeoGebra. Using the collected data, relative and absolute frequencies were calculated in relation to the number of species identified per apiary. This analysis included both the morphological characteristics and the weight in grams (g) of the collected pollen.
The taxonomy and nomenclature, as well as the threat category of the species mentioned in the study area were reviewed in the works of the Ministry of the Environment (MAE, 2013); Aguirre et al. (2014); Chimarro et al. (2023); Jaramillo et al. (2024); while, common names were provided by local guides as described by Jiménez et al. (2021).
RESULTS AND DISCUSSION
I identification of the botanical and morphological origin of the pollen from apiaries of the Quimis enclosure
The results of the microscopic analysis of the pollen load are reflected in Table 1, where 21 pollen types can be seen corresponding to species that belong to 13 plant families, distributed around the five apiaries under study in the Quimis enclosure.
Table 1. - Species inventoried in the Tropical Dry Forest of the Quimis enclosure
| N.º | Species | Family |
| 1 |
|
Convolvulaceae |
| 2 |
|
Fabaceae |
| 3 |
|
Malvaceae |
| 4 |
|
Convolvulaceae |
| 5 |
|
Muntingiaceae |
| 6 |
|
Malvaceae |
| 7 |
|
Fabaceae |
| 8 |
|
Primulaceae |
| 9 |
|
Capparaceae |
| 10 |
|
Polygonaceae |
| 11 |
|
Fabaceae |
| 12 |
|
Poaceae |
| 13 |
|
Myrtaceae |
| 14 |
|
Capparaceae |
| 15 |
|
Fabaceae |
| 16 |
|
|
| 17 |
|
Boraginaceae |
| 18 |
|
Fabaceae |
| 19 |
|
Bignoniaceae |
| 20 |
|
|
| 21 |
|
Malvaceae |
The species inventoried by family are then presented according to the morphological characteristics of the pollen, including pollen units, symmetry, apertures, exine shape, polar/equatorial shape and size.
Family Convolvulaceae
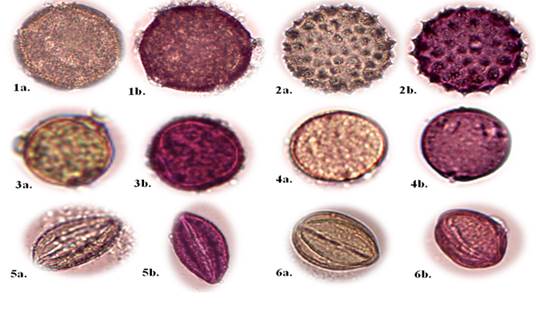
Convolvulus arvensis L. (Figure 1, No. 1a - 1b).
Botanical description: Perennial herbs with buds on the soil surface, adapted to the seasonal rainforest and Andean dry forest; simple leaves with lobed edge, triangular shape, fleshy texture; type of herbaceous stem with smooth bark (Jiménez et al., 2021). Monad-type pollen unit; grains with radiosymmetric symmetry; pantocolporado type openings; psyllate exine form; P/E shape spheroidal type; big size.
Xenostegy medium (L.) DF Austin & GW Staples (Figure 1, No. 2a -2b).
Botanical description: Herbs with stems 50 cm high, adapted in rainy seasonal dry forest and Andean dry forest; leaves simple, lobed border, cordate limb shape, of membranous texture; smooth-barked herbaceous stem type (Jiménez et al., 2021). Monad-type pollen unit; grains with radiosymmetric symmetry; apertures polypanthoporate type; form of exine equinate; shape P/E spheroidal type; large size.
Family Bignoniaceae
Handroanthus billbergii (Bureau & K. Schum.) SO, Grose (Fig. 1, Nos. 3a - 3b).
Botanical description: Large tree, compound leaves, flowers with campanulate calyx, tubular corolla (Aguirre, 2012). Monad-type pollen unit; radiosymmetric pollen grains; triporate opening; psyllate exine form; P/E shape spheroidal type; medium size.
Family Cannabaceae
Trema micrantha (L.) Bl. (Figure 1, No. 4a - 4b)
Botanical description: Evergreen tree between 5 m -13 m high, adapted to dry rainforest, Andean dry forest, lowland evergreen forest, chocó; Leaves simple, edge entire, blade shape elliptical; membranous texture (Jiménez et al., 2021). Monad-type pollen unit; bisymmetrical pollen grain; diporate opening; psyllated exine form; P/E shape spheroidal type; small size.
Family Capparaceae
Colicodendron scabridum (Kunth) Hutchinson (Figure 1, No. 5a - 5b).
Botanical description: Trees 15 m 30 m tall, adapted in rainy seasonal dry forest and Andean dry forest; leaves simple, edge entire, limb-shaped, tendrils, of coriaceous texture, tough, leather-like (Jiménez et al., 2021). Monad-type pollen unit; grains with bisymmetric symmetry; apertures of tricolpate type; form of psilate exine; shape P/E prolate-spheroidal type; small size.
Capparicordis crotonoids (Kunth) Iltis & Ram (Figure 1, No. 6a - 6b).
Botanical description: Woody plants between 2 m 5 m, adapted to dry rainforest; simple leaves, entire edge, lanceolate leaf blade shape, leathery texture, hard, leather-like (Jiménez et al., 2021). Monad-type pollen unit; grains with bisymmetric symmetry; tricolpado type openings; fossulated exine form; sub-prolate P/E form; medium size.
Family Boraginaceae
Cordia macrantha Chod (Figure 2, Nos. 7a - 7b).
Botanical description: Tree up to 15 m high, adapted in rainy seasonal dry forest and Andean dry forest, simple leaves, entire white simple flowers, gray bark (Aguirre, 2012). Pollen type unit: Monad; grains with radiosymmetric symmetry; apertures tricolporate type; reticulate exine form; form P/E prolate; small size.
Family Fabaceae
Prosopis pallida (Willd.) Kunth (Figure 2, No. 8a 8b).
Botanical Description: Small or medium-sized trees, 8 m - 15 m, adapted to seasonal rainforest and Andean dry forest; bifoliate compound leaves; entire border; linear limb shape; with a membranous texture, extremely soft (Jiménez et al., 2021). Pollen type unit: Monad; grains with radiosymmetric symmetry; tricolporate type openings; psyllate exine form; prolate P/E form; medium size.
Caesalpinia paipai Ruiz & Pav. (Figure 2, No. 9a -9b).
Botanical description: Small or medium-sized trees, 8 m 15 m, adapted to dry tropical forest; bifoliate compound leaves; leaf edge entire; leaf shape oval; membranous leaf texture, extremely soft (Jiménez et al., 2021). Pollen type unit: Monad; grains with radiosymmetric symmetry; tricolporate type openings; reticulate exine form; pollen shape oblate spheroidal; medium size.
Cassia fistula L. (Figure 2, No. 10a -10b).
Botanical description: It is a medium-sized deciduous tree, reaching 10 m in height, with a straight trunk up to 5 m and 1 m in diameter. It has pale gray bark in young specimens and dark brown in old ones. Its leaves are alternate, pinnate, with 4-8 pairs of ovate leaflets. The flowers are yellow, in pendulous clusters, with an oblong calyx and a corolla of five sub-equal petals (Bhandari et al., 2013). Pollen type unit: Monad; grains with radiosymmetric symmetry; tricolporate type openings; psyllated exine form; pollen shape spheroidal; small size.
Mimosa sensitive L. (Figure 2, No. 11a -11b).
Botanical description: Shrubs up to 3 m high with terete or subterete stems, aculeus, unguiculate and strigose. Leaves with pubescent and setose stipules, 2-9 cm petioles and oblong-elliptic to oblanceolate leaflets. Inflorescences with peduncles of 1-3.5 cm and hispid spheroid capitula. Fertile flowers, 4-mere and 4-andra, with a papiform calyx and a glabrous or puberulous corolla. Craspedia generally 2-5-articulated and hispid (Global Biodiversity Secretariat Information Facility [GBIF] 2023). Pollen type unit: Rhomboidal; grains with asymmetrical symmetry; unopened type openings; psyllated exine form; oblate pollen shape; very small size.
Leucaena leucocephala (Lam.) de Wit (Figure 2, No. 12a -12b).
Botanical description: It is a perennial, thornless shrub or small tree, up to 8 m high with deep roots. Its leaves are alternate, bipinnate, with 12 to 18 pairs of small, glabrous leaflets. The flowers are white to yellowish white, formed in axillary globose heads. The fruit is a thin, linear pod, 12 to 14 cm long, containing 15 to 30 ovate-oblong or elliptical seeds. The seeds are green when immature and dark brown when mature (Nehdi et al., 2014). Pollen type unit: monad; grains with radiosymmetric symmetry; tricolporate type openings; reticulate exine form; prolate-spheroidal pollen shape; medium size.
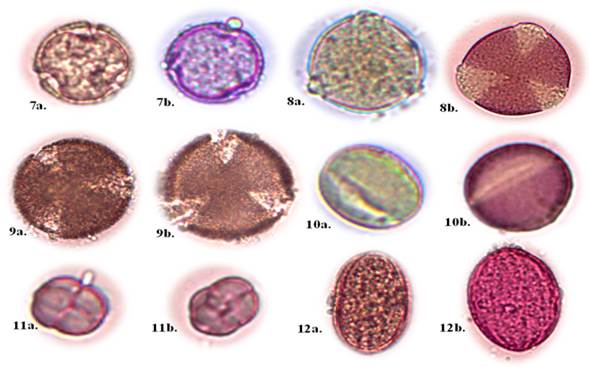 Note: (A) pollen grain without fuscin; (B) pollen grain with fuscin Family Malvaceae
Note: (A) pollen grain without fuscin; (B) pollen grain with fuscin Family MalvaceaeFig. 2. - Description of the morphological characteristics of the pollen (x400)
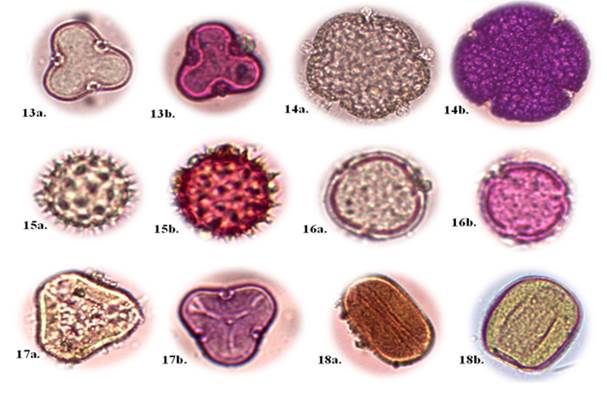
Eriotheca ruizii (K. Schum.) A. Robyns (Figure 3, No. 13a -13b).
Botanical description: Trees of 15-30 m, adapted to dry rainforest; single sheet; lobed edge; elliptical shape; with extremely soft membranous texture (Jiménez et al., 2021). Pollen type unit: Monad; grains with radiosymmetric symmetry; tricolporate type openings; reticulate exine form; oblate pollen shape; small size.
Ceiba trischistandra (A. Gray) Bakh. (Figure 3, No. 14a -14b).
Botanical description: Large trees over 30 m, adapted to seasonal rainforest and Andean dry forest; compound leaf type, palmate; entire border; limbus shape elliptical; with letterpress texture, paper or cardboard styles (Jiménez et al., 2021). Pollen type unit: Monad; grains with radiosymmetric symmetry; tetracolporate type openings; reticulate exine form; pollen shape spheroidal; big size.
Hibiscus rosa-sinensis L. (Figure 3, No. 15a -15b).
Botanical description: Tree or shrub 23 m high with branched stems. Leaves alternate, simple, ovate or ovate-lanceolate, toothed, intense green and glossy, 612 cm. Flowers 812 cm in diameter, actinomorphic, hermaphrodite, solitary, with 5 petals longer than the calyx, in colors such as intense red, white, yellow or orange. Fruit in loculicidal capsule, polyspermous, with reniform, glabrous or pubescent seeds (Greuter and Rankin, 2017). Pollen type unit: Monad; grains with radiosymmetric symmetry; pantoporate openings; echinate exine form; pollen shape spheroidal; small size.
Family Muntingiaceae
Muntingia calabura L. (Figure 3, No. 16a -16b).
Botanical description: Trees 15 m - 30 m high, adapted to dry rainforest and Andean dry forest; simple leaf type; with serrated edges; limbus shape lobed; with a fleshy texture (Jiménez et al., 2021). Pollen type unit: Monad; grains with radiosymmetric symmetry; tricolporate type openings; reticulate exine form; pollen shape spheroidal; small size.
Family Myrtaceae
Eucalyptus globulus Labill. (Figure 3, No. 17a -17b).
Botanical description: Tree that normally reaches 45 m, but can exceed 70 m. Smooth, creamy white, yellow or gray bark, which comes off in strips. Juvenile leaves sessile, opposite and glaucous; adult leaves lanceolate to narrowly lanceolate. Umbels with 1, 3 or 7 flowers. Buds turbinate or obconical; obconical, hemispherical or subglobular fruits with a level or ascending disc and 3 or 5 valves (Darriba and Pando, 2016). Pollen type unit: Monad; grains with radiosymmetric symmetry; tricolporate type openings; psliated exine form; oblate pollen shape; big size.
Family Poaceae
Zea mays L. (Figure 3, No. 18a -18b).
Botanical description: annual plant, with C4 photosynthesis, cultivated for the nutritional value of its fruits, either for human or animal consumption; cylindrical spike. The spikelets are arranged around the main axis, as in the female inflorescence; the anthers are mesiffixes. Thecae normally have longitudinal dehiscence, but there are anthers with apical pore dehiscence (Gutiérrez, 2023).
Pollen type unit: Monad; grains with bisymmetric symmetry; single-pore type openings; scabrid exine form; pollen shape oblate spheroidal; big size.
Family Polygonaceae
Triplaris cumingiana Fisch. & Mey. former CA Mey. (Figure 4, No. 19a -19b).
Botanical description: Tree 10 m -18 m high; flowers grouped in clusters 5 cm -35 cm long, red when young and then yellowish; female flowers with 3-lobed perianth; male flowers in groups of 3-5, with 6-lobed perianth, 9 stamens (Zamora and Jiménez, 2000). Pollen type unit: Monad; grains with bisymmetric symmetry; tricolporate type openings; psyllated exine form; prolate pollen shape; medium size.
Family Primulaceae
Bonellia sprucei (Mez) B. Ståhl & Källersjö (Figure 4, No. 20a -20b).
Botanical Description: Small or medium-sized trees, 8 m - 15 m, adapted to dry rainforest; type of compound leaves, odd-pinnate; whole edges; limbus shape oblanceolate; leathery, hard texture similar to leather (Jiménez et al., 2021). Pollen type unit: Monad; grains with radiosymmetric symmetry; tricolporate type openings; reticulate exine form; pollen shape sub-prolate; medium size.
Family Verbenaceae
lantana camara L. (Figure 4, No. 21a -21b).
Botanical description: Perennial woody shrub 2 m to 5 m high; leaves opposite, lanceolate; edges crenate to toothed, rough surface, axillary flowers and dense terminals (Matienzo et al., 2003). Pollen type unit: Monad; grains with bisymmetric symmetry; stephanocolpado type openings; reticulate exine form; pollen shape spheroidal; medium size.
Frequency of the polliniferous families and species most visited by Apis mellifera according to the data obtained in each apiary
The results of the relative frequency indicated that the botanical families that present the greatest interest as pollen resources for bees are Fabaceae with 23.1%, followed by Malvaceae with 14.29 %, unlike the remaining families that presented similar values between them.
The family with the largest number of honey species was the Fabaceae, in this sense the species stand out, Prosopis pallida, Caesalpinia paipai, Cassia fistula, Mimosa sensitiva, Leucaena leucocephala.
In the case of the species that resulted with a frequency of less than 6% in relation to pollen collection, it has been proven that the Apis mellifera species uses these plant species as an alternative resource when there is a low availability of floral supply. that provide a large amount of pollen in the area (Girón, 1995). This author reported that bees collect pollen from very few plant species in a proportion greater than 10 %, which can be considered as an "important food resource", while they can collect pollen from a large number of plant species, but in small proportions and these plants are called "alternative food resource"; For their part, Hidalgo et al. (1990) consider a different aspect, reporting that Apis mellifera uses only a few species as a source of pollen and that others are used occasionally, based on the selection of those species that are most advantageous.
Regarding the investigation of pollen morphology, the presence of 21 honey species corresponding to 13 families was evident, the most frequent species being Convolvulus arvensis, Ceiba trischistandra, Eriotheca ruizii, Prosopis pallida , in this same sense, the results are similar to those reported by Hidalgo et al. (1990), who recorded 21 pollen types that correspond to 13 families; Likewise, this information is close to the results obtained by Ramírez et al. (2016), who reported 17 species distributed in 13 families, the most important being Dyssodia papposa, Tithonia tubaeformis and Leucaena leucocephala, during the months of November to March in the central and northern regions of the state of Guerrero, Mexico, despite these results differ from Girón (1995), who reported 91 pollen types of which the most frequent was the species Coffea arabica within the municipality of Salgar in Antioquia, Colombia.
In the order of the previous ideas, the specific morphological characteristics of the pollen grains stand out; in the Fabaceae family, monad-type pollen predominates. Regarding shape, prolate and oblate-spheroidal morphologies prevailed, followed by oblate and prolate-spheroidal. In terms of openings, the tricolporada was the most common observed. Regarding the exine, predominantly psyllate and reticulate morphologies were identified. These results coincide with the reports of Ventura and Huamán (2008), who reported similar morphological characteristics in species of the Fabaceae family, all of which corroborates what was described by Ferreira et al. (2013), who reported the monad type as the most frequent among the pollen grains studied.
Within the categories that address the morphological characteristics of pollen, symmetry, which determines the polar view of the grain, showed a higher frequency for radiosymmetric symmetry, followed by bisymmetric symmetry, which was observed in approximately six species. Only one species presented asymmetry, which means that it does not exhibit any plane of symmetry. These results agree with the research of Ferreira et al. (2013), who, in their study of 1668 species, concluded that in nature, isopolar and radioisometric pollen grains are the most common, followed by radioisometric apolar and bilateral heteropolar, although in a smaller proportion.
Following the observations made, this research represents the first morphological study of pollen in the tropical dry forest of the Quimis enclosure, which adds important value to the understanding of the vegetation in the area. Furthermore, it has been compared with previous works carried out in Ecuador and various regions of Latin America, as is the case of Ferreira et al. (2013).
In the case of the most frequent pollen resources in the five apiaries under study, it was found that they come from the Convolvulus arvensis, Eriotheca ruizii, Ceiba trischistandra, Prosopis pale with 8.3 %. In that same context, the other species present a low percentage, however, these plants somehow manage to be used by the Apis mellifera species. as an alternative pollen resource and this happens when there is a low availability of species that provide a large amount of pollen around the apiaries.
In reference to the above, Ramírez et al. (2016) maintain that the floral origin of nectar, from which bees produce honey, and pollen loads, are a crucial source of protein for the colony, which can be identified through palynological analyses. On the other hand, Hidalgo et al. (1990) highlight the importance of understanding the relationship between insects and plants, since this relationship sheds light on the feeding preferences of bees. In this way, by knowing the vegetation of a region, the beekeeping potential of an area can be evaluated.
Frequency of weight in (g) of pollen collected per month according to the number of species identified by each apiary
In Table 2, the weight of pollen is shown as an independent variable while the number of species is considered as the dependent variable and reflects the relationship between the number of species and the weight in grams (g) of pollen collected throughout three months of observation in each of the apiaries.
Table 2. - Amount of weight in g per number of species per apiary of the Quimis enclosure
| Weight (g) | No. of species per apiary |
| 44 | 11 |
| 15 | 11 |
| 42 | 15 |
| 27 | 14 |
| 24 | 9 |
As seen in Table 2, the pollen weights range between 15 g and 44 g, and the number of species ranges from 9 to 15. These data were analyzed and a trend line was obtained that corresponds to a polynomial of degree 3, with a coefficient of determination R2 of 0.907. This suggests a significant relationship between pollen weight and species diversity, indicating that as the weight of pollen collected increases, the number of species present in the apiaries tends to increase (Figure 5).
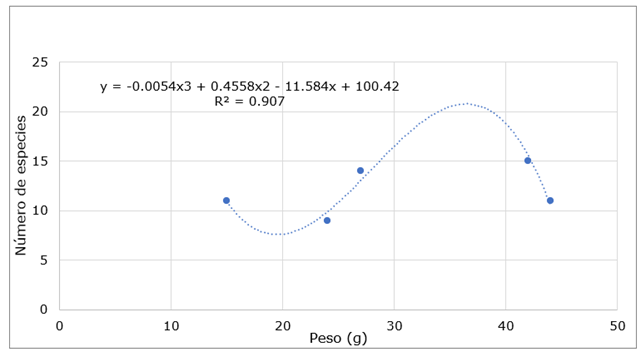
Fig. 5. - Calculation of Polynomial degree 3 in relation to the weight of the pollen grain of the honey species of the Tropical Dry Forest of the Quimis enclosure
By calculating a polynomial of degree 3 related to the weight of the pollen grain in the Tropical Dry Forest of the Quimis enclosure, it has been possible to obtain a tool that provides a more precise understanding of the relationship between the weight of the pollen and the amount of species present in the study area. This model allows us to estimate both the minimum and maximum number of species that could exist based on the weight of the pollen. The results show a fit to a polynomial model of degree 3, with a coefficient of determination (R2 = 0.907). This high value of R2 suggests that the polynomial model is a good representation of the relationship between pollen weight and the number of species, indicating that approximately 90.7% of the variability in the number of species can be explained by the variation in pollen weight, which reinforces the validity of the model Equations 1, 2, 3 and 4.
Applying differential calculus of a real variable Equations 1, 2, 3 and 4:
The global extremes can be obtained in the definition domain of interest, the calculated global minimum value is 19.52 g and approximately eight species and the maximum value is 36.45 g and approximately 21 species. These findings indicate that, in the investigated area, the estimate of the number of honey species is in an approximate range of 8 to 21.
In summary, the global extremes of the cubic trend function have been identified, which provides valuable information on the relationship between pollen weight and species diversity in the study area.
This study highlights the importance of statistical and mathematical analysis to understand the complex biological relationships in bee ecology, all of which highlights the crucial role of pollen weight in species diversity, offering relevant information for decision making in apiary management and biodiversity conservation. Furthermore, it contrasts with previous studies, such as that of Jáuregui (2011), which used different methods and approaches to evaluate pollen yield and its impact on the diversity of honey species.
Frequency based on the morphological characteristics identified in the pollen of each honey species
Table 3 shows the number of honey species based on morphological characteristics of the pollen. Categories studied include pollen units, symmetry, apertures, exine shape, pollen shape and size. Each row of the table presents both the Absolute Frequency (FA) and the Relative Frequency (FR), in addition to showing the Accumulated Absolute Frequency (FAA) and the Accumulated Relative Frequency (FRA).
Table 3. - Number of honey species according to the morphological characteristics of the pollen
| pollen units | FA | FR (%) | FAA | FRA |
| Monad | twenty | 0.95 | twenty | 0.95 |
| Rhomboid | 1 | 0.05 | 21 | 1.00 |
| Symmetry | - | - | - | - |
| Asymmetric | 1 | 0.05 | 1 | 0.05 |
| Bisymmetric | 6 | 0.29 | 7 | 0.33 |
| Radiosymmetric | 14 | 0.67 | 21 | 1.00 |
| Openings | - | - | - | - |
| Diporados | 1 | 0.05 | 1 | 0.05 |
| Estefanocolpado | 1 | 0.05 | two | 0.10 |
| Unopened | 1 | 0.05 | 3 | 0.14 |
| Monoporated | 1 | 0.05 | 4 | 0.19 |
| Pantocolporate | 1 | 0.05 | 5 | 0.24 |
| Pantoporado | 1 | 0.05 | 6 | 0.29 |
| Polypantoporate | 1 | 0.05 | 7 | 0.33 |
| Tetracolporate | 1 | 0.05 | 8 | 0.38 |
| Tricolpado | 2 | 0.10 | 10 | 0.48 |
| Tricolporate | 10 | 0.48 | twenty | 0.95 |
| Triporate | 1 | 0.05 | twenty-one | 1.00 |
| exine form | - | - | - | - |
| equined | 2 | 0.10 | 2 | 0.10 |
| Scabrida | 1 | 0.05 | 3 | 0.14 |
| Fossulated | 1 | 0.05 | 4 | 0.19 |
| Psilada | 9 | 0.43 | 13 | 0.62 |
| Reticulated | 8 | 0.38 | 21 | 1.00 |
| Form of the pollen | - | - | - | - |
| Spheroidal | 8 | 0.38 | 8 | 0.38 |
| Spherical | 1 | 0.05 | 9 | 0.43 |
| By offering | 3 | 0.14 | 12 | 0.57 |
| spheroidal offering | 2 | 0.10 | 14 | 0.67 |
| Pronounced | 3 | 0.14 | 17 | 0.81 |
| Prolate-spheroidal | 2 | 0.10 | 19 | 0.90 |
| Sub-prolate | 2 | 0.10 | twenty-one | 1.00 |
| Size | - | - | - | - |
| Big | 5 | 0.24 | 5 | 0.24 |
| Medium | 8 | 0.38 | 13 | 0.62 |
| Very small | 1 | 0.05 | 14 | 0.67 |
| Little | 7 | 0.33 | twenty-one | 1.00 |
Note: Absolute frequency (FA); Relative frequency (FR); Accumulated absolute frequency (FAA); Cumulative relative frequency (FRA)
According to the results of Table 3, specifically, in the category of pollen units, it stands out that 0.95% corresponds to monad type species. In relation to symmetry, 0.67 % are characterized by being radiosymmetric, while 0.48% have tricolporate openings. Regarding the shape of the exine, 0.43 % exhibit a psyllate shape, and 0.38% show a spheroidal shape. Finally, when it comes to size, 0.38 % are classified as medium-sized.
It can also be seen that families with two or more species were analyzed based on their morphological characteristics to evaluate possible relationships between them. Among the families studied, Fabaceae stood out by presenting five polliniferous species, followed by Malvaceae with three species, on the other hand, the Capparaceae and Convolvulaceae families presented only two species each.
It is relevant to highlight that, in most of the characters, the species of each family presented significant differences, except in pollen units and symmetry, where greater uniformity was observed. In this order of ideas, the two species of the Convolvulaceae family shared the same pollen size, while the two species of the Capparaceae family exhibited the same type of openings.
CONCLUSIONS
The study in five apiaries of the tropical dry forest of Quimis revealed 21 honey species from 13 families and 21 genera, especially Ceiba trischistandra, Convolvulus arvensis, Eriotheca ruizii and Prosopis pale.
Morphological differences in pollen were observed between species of the same family, except in the pollen unit and symmetry.
REFERENCIAS BIBLIOGRÁFICAS
AGUIRRE, Z., 2012. Especies forestales de los bosques secos del Ecuador [en línea]. Primera. S.l.: FAO-ONU-REDDEditor: MAE. Disponible en: https://www.researchgate.net/publication/280625434_Especies_forestales_de_los_bosques_secos_del_Ecuador. [ Links ]
AGUIRRE MENDOZA, Z., BURI SIVISACA, D., GEADA LÓPEZ, G., & BETANCOURT FIGUERAS, Y. 2014. Composición florística, estructura y endemismo en una parcela permanente de bosque seco en Zapotillo, provincia de Loja, Ecuador. Loja: Universidad Nacional de Loja. [en línea] Disponible en: https://dspace.unl.edu.ec/jspui/handle/123456789/10306 [ Links ]
BHANDARI, S.S., KHURANA, K., BALYAN, A., KABRA, M.P., y NEGI, K. 2013. Una revisión sobre la fístula de Cassia.Revista asiática de investigación y desarrollo farmacéutico, 217-219. [ Links ]
CHAMORRO, F. J., LEÓN, D., & NATES, G. 2013. El polen apícola como producto forestal no maderable en la cordillera oriental de Colombia. Colomb. for. [online]. 2013, vol.16, n.1. ISSN 0120-0739. Disponible en: http://www.scielo.org.co/scielo.php?pid=S0120-07392013000100004&script=sci_arttext [ Links ]
DARRIBA, A.F., y PANDO, F.J.S., 2016. El Género Eucalyptus (Myrtaceae) en Galicia: Claves y descripción. Nova Acta Científica Compostelana [en línea], vol. 23, ISSN 2340-0021. Disponible en: https://revistas.usc.gal/index.php/nacc/article/view/2962. [ Links ]
DE BOADA, D. O., PARRA, G. N., & BUSTOS B., I. 1987. Procedencia Botánica del Polen Almacenado por Apis mellifera, en alrededores de la Sabana de Bogotá. Agronomía Colombiana, [en línea] vol. 7, pp. 31-38. Disponible en: https://revistas.unal.edu.co/index.php/agrocol/article/download/20919/21827/0 [ Links ]
DE BOADA, D. O., & COGUA, J. 1989. Reconocimiento de granos de polen de algunas plantas melíferas en la Sabana de Bogotá. Agronomía Colombiana, [en línea] vol. 6 no. 1-2, 52-63. Disponible en: https://revistas.unal.edu.co/index.php/agrocol/article/view/20997 [ Links ]
FERREIRA, Y. A., FIGUEROA, W., & BAYONA, Y. M. 2013. Morfología Polínica de especies arbóreas predominantes de San José de Cúcuta. Mundo FESC. Disponible en: https://dialnet.unirioja.es/servlet/articulo?codigo=4966235 [ Links ]
GIRÓN, M. 1995. Analisis palinológico de la miel y la carga de polen colectada por Apis mellifera en el Suroeste de Antioquia, Colombia. Disponible en: https://bibliotecadigital.univalle.edu.co/server/api/core/bitstreams/4037e7dd-d97a-4c4a-b8bc-109d0b9c7fd0/content [ Links ]
GREUTER, W., & RANKIN, R. 2017. Plantas vasculares de Cuba. Inventario preliminar.Botanischer Garten und Botanisches Museum Berlin. Berlín, Alemania. [ Links ]
GUTIÉRREZ, H. 2023.Botánica sistemática de las plantas con semillas 2: principales familias de Gimnospermas y Monocotiledóneas. Universidad Nacional del Litoral. ISBN: 978-987-657-959-9 Disponible en: https://bibliotecavirtual.unl.edu.ar:8443/handle/11185/7127 [ Links ]
HIDALGO, M. I., BOOTELLO, M. L., & PACHECO, J. 1990. Origen floral de las cargas de polen recogidas por Apis mellifera L. en Alora (Malaga, España). Acta Botánica Malacitana [en línea] vol. 15, 33-44. Disponible en: https://dialnet.unirioja.es/descarga/articulo/3012964.pdf [ Links ]
INSUASTY-SANTACRUZ, E., MARTÍNEZ-BENAVIDES, J., & JURADO-GÁMEZ, H. 2017. Determinación melisopalinológica de miel de abejas Apis mellifera producida con flora de clima frío, principalmente Trifolium repens L.Revista Veterinaria y Zootecnia (On Line), [en línea] vol. 11 no. 1, pp. 74-82. Disponible en: https://revistasojs.ucaldas.edu.co/index.php/vetzootec/article/view/3389 [ Links ]
JARAMILLO, P., & DEL MAR TRIGO, M. 2011. Guía rápida de polen de las Islas Galápagos. Málaga, España: Fundación Charles Darwin. [online]. Disponible en: https://www.researchgate.net/publication/261988309_Guia_Rapida_del_Polen_de_las_Islas_Galapagos [ Links ]
JARAMILLO DIAZ, NELSON; AGUIRRE MENDOZA, ZHOFRE., y YAGUANA PUGLLA, CELSO. 2024. Componente florístico del bosque seco, sector Bramaderos, parroquia Guachanama, cantón Paltas, suroccidente de la provincia de Loja, Ecuador. Arnaldoa [online]. 2018, vol.25, n.1 pp.87-104. ISSN 1815-8242. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S2413-32992018000100005&lng=es&nrm=iso>. http://dx.doi.org/http://doi.org/10.22497/arnaldoa.251.25105. [ Links ]
MATIENZO, Y., RAMOS, B. y RIJO, E., 2003. Revisión Bibliográfica Sobre Lantana Camara L. Una Amenaza Para La Ganadería. Fitosanidad [en línea], vol. 7, no. 4, ISSN 1562-3009, 1818-1686. Disponible en: https://www.redalyc.org/articulo.oa?id=209118173010. [ Links ]
MÉNDEZ, V. M., SÁNCHEZ, A. C., & CONCEPCIÓN LUPO, L.2021. Caracterización de los recursos tróficos utilizados por Apis mellifera L. en un área de las Yungas en el norte de Salta (Argentina). Boletin de la Sociedad Argentina de Botánica, [en línea] 2-16. Disponible en: doi: https://doi.org/10.31055/1851.2372.v56.n2.29926 [ Links ]
MINISTERIO DEL AMBIENTE DEL ECUADOR. MAE, 2013. Sistema de clasificación de los ecosistemas del Ecuador continental. Subsecretaría de Patrimonio Natural: Quito. [en línea] Disponible en: https://www.ambiente.gob.ec/wp-content/uploads/downloads/2012/09/LEYENDA -ECOSISTEMAS_ECUADOR_2.pdf [ Links ]
MONTOYA, P. M., LEÓN-BONILLA, D., & NATES-PARRA, G. 2014. Catálogo de polen en mieles de Apis mellifera provenientes de zonas cafeteras en la Sierra Nevada de Santa Marta, Magdalena, Colombia. Revista Académica Colombia, [en línea] vol. 38 no.1 pp., 364-84. Disponible en: http://www.scielo.org.co/scielo.php?pid=S037039082014000400003&script=sci_ [ Links ]
NEHDI, I.A., SBIHI, H., TAN, C.P., y AL-RESAYES, S.I. 2014. Aceite de semilla de Leucaena leucocephala (Lam.) de Wit: Caracterización y usos.Cultivos y Productos Industriales,52, 582-587. [ Links ]
ORGANIZACIÓN DE LAS NACIONES UNIDAS PARA LA ALIMENTACIÓN Y LA AGRICULTURA. 2018. FAO. La importancia de las abejas en la biodiversidad y su contribución a la seguridad alimentaria y nutricional: FAO [en línea] Disponible en: https://www.fao.org/guinea-ecuatorial/noticias/detail-events/en/c/1133248/ [ Links ]
SAYAS, R., & HUAMÁN, M. L. 2009. Determinación de la flora polinífera del valle de Oxapampa (Pasco-Perú) en base a estudios Palinológicos. Ecología aplicada, [online] vol. 8 no. 1-2, pp. 53-59. Disponible en: doi: http://dx.doi.org/10.21704/rea.v8i1-2.382 [ Links ]
SECRETARÍA DE GLOBAL BIODIVERSITY INFORMATION FACILITY [GBIF] 2023. Mimosa sensible var. sensible. En Taxonomía de la columna vertebral de GBIF. Conjunto de datos de lista de verificación. SECRETARÍA DE GLOBAL BIODIVERSITY INFORMATION FACILITY [GBIF] Disponible en: https://doi.org/10.15468/39omei. [ Links ]
SIERRA, D.J.J., 2011. Estudio de la productividad de polen y miel de abeja (Apis mellifera), utilizando trampas caza polen con diferentes períodos de estadía en las Colmenas, en San Cristobal de Caranqui, provincia de Imbabura. AXIOMA [en línea], no. 7, ISSN 2550-6684. Disponible en: https://axioma.pucesi.edu.ec/index.php/axioma/article/view/343. [ Links ]
JIMÉNEZ GONZÁLEZ, A.J., LOOR, M.J.C., SALAZAR, L.M.V., y BLANDARIZ, S.R., 2021. Caracterización de las especies melíferas en el bosque seco tropical orientada a su conservación. Revista Cubana de Ciencias Forestales [en línea], vol. 9, no. 3, ISSN 2310-3469. Disponible en: https://cfores.upr.edu.cu/index.php/cfores/article/view/701. [ Links ]
RAMÍREZ-ARRIAGA, ELIA; MARTINEZ-BERNAL, ANGÉLICA; RAMIREZ MALDONADO, NADIA., y MARTINEZ-HERNANDEZ, ENRIQUE. 2016 Análisis palinológico de mieles y cargas de polen de Apis mellifera(Apidae) de la región Centro y Norte del estado de Guerrero, México. Bot. sci ISSN 2007-4476. [online]., vol.94, n.1, pp.141-156. Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-42982016000100141&lng=es&nrm=iso. https://doi.org/10.17129/botsci.217. [ Links ]
VELANDIA, M., RESTREPO, S., CUBILLOS, P., APONTE, A., & SILVA, L. M. 2012. Catálogo fotográfico de especies de flora apícola en los departamentos de Cauca, Huila y Bolviar. Instituto Humboldt, [online] 84. Disponible en: http://repository.humboldt.org.co/bitstream/handle/20.500.11761 /31379/199.pdf?sequence=1&isAllowed=y [ Links ]
VENTURA, K., & HUAMÁN, L. 2008. Morfología polínica de la familia Fabaceae de la parte de baja de los valles de pativilca y fortaleza (Lima-Perú). The Biologist, [online] 112-134. Disponible en: https://dialnet.unirioja.es/servlet/articulo?codigo=3989213 [ Links ]
ZAMORA, N., & JIMÉNEZ, L. 2000. Árboles de Costa Rica, Centro Científico Tropical. Conservación Internacional y Instituto Nacional de Biodiversidad, 374. [ Links ]
Received: January 16, 2024; Accepted: March 17, 2024











 texto en
texto en