Introduction
Highly pathogenic avian influenza (HPAI) viruses are considered a resident enzootic crisis threatening global, local economy and health conditions. For almost 16 years, the HPAI viruses have gone through several genetic variations that, in turn, subjected us to different emerging strains throughout different countries.1 In 2010, the H5N8 clade 2.3.4.4 strain was first detected in some types of wild migratory birds in Asia and then spread worldwide.2
By the end of 2016, the H5N8 strain was first reported in Egypt and has become endemic, which has been enhanced by the geographical location of Egypt as an articulating region between three continents. This unique location places Egypt in the crossing ways of wild migratory birds from various destinations of the world.3 The H5N8 virus of clade 2.3.4.4 was considered highly pathogenic, so the Egyptian government started to implement control plans based on biosafety and biosecurity programs; this was carried out by increasing public awareness, culling infected birds, the sanitary burial of dead carcasses, and prohibition of backyard rearing, in addition to the limitation of commercial movements of birds between governorates. Vaccination programs were the first preventive and protective measure applied to protect against infection and limit disease spreading by minimization of viral shedding.4
New strains of HPAI virus H5N8 are recognized almost annually, causing tragic economic losses in the Egyptian poultry industry sector. They have resulted from wide variation in the hemagglutinin segment of the virus, in association with antigenic variation in the same subtype, provoking new reassortant strains that challenge the protective ability of permitted vaccination programs.5 Therefore, regular follow-up of the efficacy of commercially used vaccines against new isolates is very critical to avoid a crisis of high morbidity and mortality.6
In this study, an experiment was designed to monitor the immunological response of vaccinated specific pathogen free (SPF) chickens by hemagglutination inhibition test (HI) and challenge test; the SPF chickens were vaccinated with some widely used commercial inactivated avian influenza vaccines (AIV) formulated from different H5 strains and an inactivated recombinant baculovirus vectored vaccine expressing H5&NDv (Newcastle disease virus). The HI test was done using two Egyptian HPAI H5N8 isolates of clade 2.3.4.4b, A/chicken/Egypt/1526v/2020/H5N8 (H5N8-CH) and A/Duck/Egypt/Qalubia321/2021 (H5N8-D), while the challenge experiment was performed using only the H5N8-D strain under strict hygienic measures including isolators.
Material and Methods
Specific pathogen free chicks and eggs
SPF embryonated chicken eggs (ECE) were obtained from Koum Oshiem SPF chicken farm, Fayoum, Egypt. They were used for virus titration and shedding.7 One-day-old SPF chicks (total number 480) were raised in HEPA-filtered isolators with controlled lighting, feed, and water-supplied adequately. They were used to determine virus lethal dose 50 (LD50) and monitoring the potency and efficacy of the tested inactivated vaccines.
Vaccines
Different eight inactivated commercial AIV were kindly supplied by the Central Laboratory for Evaluation of Veterinary Biologics (CLEVB) Abbassia- Cairo (Table 1). Seven inactivated whole avian influenza (AI) virus vaccines were classic oil-adjuvanted inactivated vaccines with different seed viruses, the eighth vaccine was a bivalent inactivated whole virus vaccine from the inactivated recombinant baculovirus vector-H5AI, propagated in insect cells and Newcastle disease virus.
Table 1 Types of inactivated AI-H5 vaccines used in the experiment.
| Name | Strain | Type | Lineage | Similarity to H5N8 challenge virus |
|---|---|---|---|---|
| Reassortant avian influenza virus (Re5-H5N1) | A/duck/Anhui/1/2006 (H5N1) | Imported inactivated reassortant | 2.3.4 | 98% |
| Volvac B.E.S.T (rBac-H5+ND) | A/duck/china/E319-2/2003 (H5N1) and Lasota | Imported inactivated recombinant baculovirus -AI + ND | 2.3.2 | 93.3% |
| Egy flu (Egy-H5N1) | RGA/chicken/Egypt/18-H/2009 (H5N1) | Imported inactivated reassortant | 2.2.1.1 | 86.8% |
| Poulvac FluFendi AI (Re-H5N3) | A/chicken/Vietnam/c58/2004 (H5N3) | Imported inactivated reassortant | Clade I | 91.3% |
| Nobilis Influenza H5N2 (Pot-H5) | A/duck/Potsdam/1402-6/1986 (H5N2) | Imported inactivated LPAIV | Eurasian | 81.8% |
| OPTIMUNE Avian Influenza vaccine (Mex-H5) | A/chicken/Mexico/232/1994 (H5N2) | Imported inactivated LPAIV | North American | 75.6% |
| MEFLUVAC H5 PLUS 8 (Loc1-H5N1+H5N8) | A/chicken/Egypt/RG-13CAL/2017 (H5N1) A/chicken/Egypt/ME1010/2016 (H5N1) A/chicken/Egypt/ME-2018 (H5N8) | local inactivated reassortant | 2.2.1.2 2.2.1.1 2.3.4.4b | 88.3% 86.7% 98.5 % |
| Avian Flu H5 plus (Loc2-H5N8) | A/chicken/Egypt/D10552B/2015 (H5N8) + A/green winged teal/Egypt/877/2016 | local inactivated reassortant | 2.3.4.4b | 83.3% 98% |
LPAIV: Low pathogenic avian influenza virus. ND: Newcastle disease.
Viruses
Two different HPAI H5N8 viruses were locally isolated and were sent to be identified and sequenced by the Reference Laboratory for Veterinary Quality Control on Poultry Production (RLQP), Animal Health Research Institute (AHRI) -DOKKI- GIZA were used:
- A/chicken/Egypt/1526v/2020/H5N8 (H5N8-CH), chicken origin, identified as clade 2.3.4.4b.
- A/Duck/Egypt/Qalubia321/2021 (H5N8-D), duck origin, identified as clade 2.3.4.4b; its percentage similarity to different vaccine strains is shown in Tab1e 1.
The (H5N8-D) isolate was used for serology tests, challenge experiments, and shedding tracing, while (H5N8-CH) isolate was used for serology tests.
Virus titration in specific pathogen free eggs
Serial tenfold virus dilution (10-⁵ to 10-12) of the virus in sterile antibiotic saline was inoculated in five ECE via allantoic sac (0.1 mL/egg). The inoculated embryos were incubated at 37oC-38oC and candled twice, daily for 6 days. Slide hemagglutination test (HA) was applied to the allantoic fluid of inoculated chicken embryos to detect positive HA reaction. The 50% egg infective dose (EID50) was estimated using the Reed and Muench method.8
Virus titration in chickens
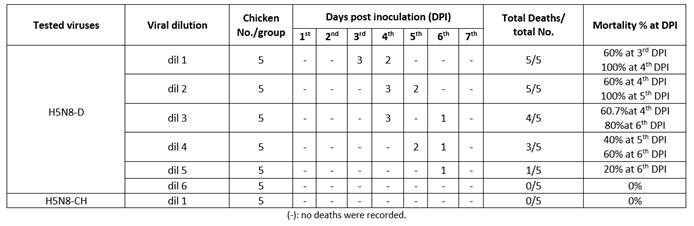
It was done according to World Organization for Animal Health (WOAH)9 for each viral isolate. Serial tenfold dilution (10-1: 10-6) of each H5N8 isolate was done. Each dilution was injected into five SPF chickens, 0.1 mL/bird. Daily deaths were recorded for one week to calculate viral LD50 using Reed and Muench method.8
Potency test
According to WOAH9 specifications, 4 week old SPF chickens, were vaccinated subcutaneously (S/C) with the field dose recommended by the companies that produce the inactivated vaccines listed in Table 1. Blood samples were collected weekly post-vaccination and serum samples were separated, inactivated at 56oC/30 minutes, and stored at -20oC until used. Serological analysis to determine the level of antibodies against H5 was performed by the hemagglutination inhibition (HI) test using H5N8-CH and H5N8-D isolates. At 4 weeks post-vaccination (wpv), subgroups from the vaccinated and control groups were challenged with HPAI H5N8-D virus to determine the protection percentage of the tested vaccines. The challenge dose (109 EID50) was inoculated intranasally (0.1 mL/each bird). Chickens were observed daily for 10 days after challenge. All dead and clinically infected birds were recorded as shown in Table 4. Tracheal and fecal swabs were taken 2 days post-infection (dpi) from all groups to estimate the viral shedding reduction using SPF ECE according to WOAH.9 The neutralization index (NI) was calculated by subtracting the virus titer of vaccinated SPF chickens from the virus titter of control SPF chickens. The NI should be ≥ 2 according to WOAH.9
Experimental design
A total of 225 SPF chickens were divided into nine groups (25 chickens/group). Eight groups were numbered from 1 to 8, each group was vaccinated with one of the tested vaccines shown in Table 1. The ninth group remained unvaccinated as control group. After 4 weeks of vaccination, the nine groups were subdivided into two subgroups A and B. Subgroup A contains 10 chickens per vaccination group. Subgroup A was subjected to the challenge test to estimate the efficacy of the AIV tested against the HPAI H5N8-D virus. While subgroup B of each experimental group remained unchallenged and was monitored weekly for serological analysis of immune response of the vaccinated with the different tested AI vaccines until the end of the experiment at the 11th wpv.
Ethical approval
All animal experiments in this study were conducted in strict compliance and adherence to the relevant policies regarding animal handling as mandated by international, national, and/or institutional guidelines for animal care, and were approved by the Research Ethical Committee at the National Research Center, Cairo, Egypt.
Results and Discussion
Since 2006, the AI virus has been threatening the poultry industry in Egypt. Despite the great effort carried out by governmental authorities to apply strict vaccination programs to control the disease, it has not been possible to completely eliminate the virus from the poultry field.10 Until now, novel strains have been isolated periodically due to persistent viral mutation. Recently, the 2.3.4.4 clade isolated from ducks was found to be the most predominant since 2016.5
This study was carried out to evaluate the ability of the most recently used licensed commercial inactivated AIV in Egypt (shown in Table 1) to protect chickens against two isolated H5N8 AI virus, as well as the influence of the percentage similarity between the challenge strain and the different vaccine strains on the immune response.
Serological analysis using HI and cross HI were carried out, in addition to challenge test,9 to evaluate the commercial AIVs. The HI test and cross HI antibody titers were monitored weekly post-vaccination using H5N8-CH and H5N8-D isolates (Table 2). All the imported vaccines induced low cross HI antibody titer against H5N8-D isolate at the first 3 wpv ranged from 1.4 to 6.8 log2. After that, the antibody titers induced by all imported vaccines started to show a slight increase until the 6th wpv, this low level of antibodies ranging from 4.5 to 6.8 log2. Thereafter, the antibody titer gradually decreased until the 11th wpv reaching 3.5 to 5.5 log2 for the imported vaccines tested. The same results were noticed for the cross HI antibody titers against H5N8-CH as shown in Table 2.
The locally prepared vaccines (loc1-H5N1+H5N8 and loc2-H5N8) induced higher levels of HI antibody titers against H5N8-D isolate reaching 7.8 and 7.5 log2 at 5th wpv; in the case of the H5N8-CH isolate, the HI antibody titer reached its peak at 6th wpv achieving 8 and 6.5 log2 (Table 2). Also, the HI antibody titer against H5N8-D antigen began to decrease from the 6th wpv to reach 6.8 and 6 log2 at the 11th wpv; while for H5N8-CH antigen it started to decline at 7th wpv, to reach 7 and 5.5 log2 at 11th wpv for the two local vaccines, respectively.
Table 2 Mean HI antibody titer of different inactivated AI-H5 vaccines using local H5N8-D and H5N8-CH antigens.
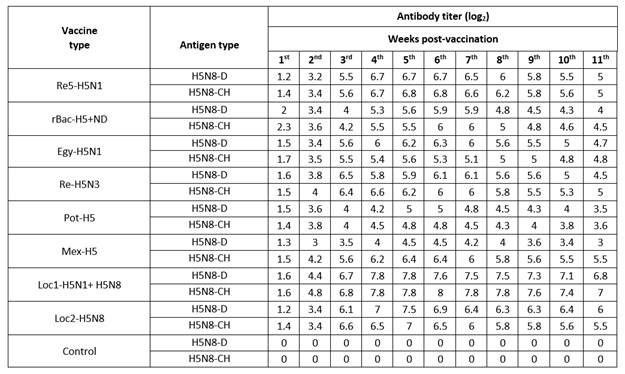
From the previous data, it is observed that the HI antibody titer achieved by the locally prepared vaccines against H5N8-D and H5N8-CH antigens was higher than the cross HI antibody titers induced by all imported vaccines against the heterologous H5N8 antigens. These relatively low antibody titers in the cross HI test were expected due to genetic and antigenic differences in the HA gene between HPAI H5N8 antigens and the different vaccine strains of the tested imported AlV compared to the homologous HI test results in the case of local AlV.11
The efficacy of the tested AI inactivated vaccines was evaluated using HPAI H5N8 clade 2.3.4.4b at 4 wpv.9
When the viral titer and pathogenicity of the two viral isolates H5N8-D and H5N8-CH were tested, it was found that the EID50 were 109 and 1010 for H5N8-CH and H5N8-D, respectively, while LD50 in chicken was 105.2/mL for H5N8-D isolate, but H5N8-CH isolate was not lethal.
The HA titer was 28 and 27 for H5N8-CH and H5N8-D, respectively. As the H5N8-CH isolate was not lethal, the challenge doses were determined depending on pathogenicity of H5N8-D in chickens (Table 3) that could infect 100% of susceptible chickens within 3 to 4 dpi and it was 104.2 LD50 or 109 EID50. This agrees with12 who stated that LD50 of the circulating AI virus was very low in comparison to the previously isolated AI strains.
Table 4 shows the protection percentage of all Al vaccines tested against the H5N8-D virus. It was observed that 100% of chickens in groups vaccinated with Re-H5N1, rBac-H5+ND, Re-H5N3 and Loc1-H5N1+ H5N8 vaccines survived the challenge with HPAI H5N8-D, while the Egy-H5N1, Pot-H5, Mex-H5 and loc2-H5N8 vaccines protected 90% of chickens against the same challenge virus. All the non-vaccinated chickens showed severe clinical signs with 100% mortality at 4-day post challenge (dpc) against the challenge dose 109 EID50 (0.1mL/bird) of HPAI H5N8-D virus.
Although few reports indicated that HPAI H5N8-D viruses induced asymptomatic disease in ducks with prolonged virus shedding,13 an increased viral adaptation to chicken was observed within the HPAI of 2.3.4.4.b clade viruses.14 This was supported by the findings that the HPAI H5N8 challenge group showed typical AI signs and 100% mortality for the H5N8-D isolate and not for H5N8-CH isolate.
Also, there was a reduction in the viral shedding from the challenged vaccinated chicken groups. The NI 5, 5, 7, 6, 4, 4, 6 and 5 for the AI vaccines are listed in Table 4, respectively. The NI should be ≥ 2 according to WOAH.9
From the previous results it was observed that despite the relatively low HI antibody titers against H5N8-D virus achieved by the imported commercial AI vaccines, formulated from different H5 seed virus strains of clade 2.3.4 (Re5-H5N1), clade 2.3.2 (rBac-H5+ND) and clade I (Re-H5N3), there was a high protection percentage reaching 100% in the vaccines and a reduction of viral shedding titers against the same virus with a range of 5:6 log10 EID50.
Table 4 Efficacy of different inactivated AI-H5 vaccines in chicken challenged with HPAI H5N8-D virus.
| Name | Strain | Type | Lineage | Similarity to H5N8 challenge virus |
|---|---|---|---|---|
| Reassortant avian influenza virus (Re5-H5N1) | A/duck/Anhui/1/2006 (H5N1) | Imported inactivated reassortant | 2.3.4 | 98% |
| Volvac B.E.S.T (rBac-H5+ND) | A/duck/china/E319-2/2003 (H5N1) and Lasota | Imported inactivated recombinant baculovirus -AI + ND | 2.3.2 | 93.3% |
| Egy flu (Egy-H5N1) | RGA/chicken/Egypt/18-H/2009 (H5N1) | Imported inactivated reassortant | 2.2.1.1 | 86.8% |
| Poulvac FluFendi AI (Re-H5N3) | A/chicken/Vietnam/c58/2004 (H5N3) | Imported inactivated reassortant | Clade I | 91.3% |
| Nobilis Influenza H5N2 (Pot-H5) | A/duck/Potsdam/1402-6/1986 (H5N2) | Imported inactivated LPAIV | Eurasian | 81.8% |
| OPTIMUNE Avian Influenza vaccine (Mex-H5) | A/chicken/Mexico/232/1994 (H5N2) | Imported inactivated LPAIV | North American | 75.6% |
| MEFLUVAC H5 PLUS 8 (Loc1-H5N1+H5N8) | A/chicken/Egypt/RG-13CAL/2017 (H5N1) A/chicken/Egypt/ME1010/2016 (H5N1) A/chicken/Egypt/ME-2018 (H5N8) | local inactivated reassortant | 2.2.1.2 2.2.1.1 2.3.4.4b | 88.3% 86.7% 98.5 % |
| Avian Flu H5 plus (Loc2-H5N8) | A/chicken/Egypt/D10552B/2015 (H5N8) + A/green winged teal/Egypt/877/2016 | local inactivated reassortant | 2.3.4.4b | 83.3% 98% |
*TS: tracheal swap; FS: fecal swap; VT: viral titer (log EID50); NI: neutralization index; -ve: absence of virus particles; +ve: presence of virus particles; (-): no deaths were recorded.
More over other imported vaccines as Pot-H5 and Mex-H5 showed lower protection levels against the H5N8-D challenge virus reaching 90% with low viral shedding reduction of 104 EID50. This might be due to the lower similarity between the vaccinal strain and the challenge HPAI H5N8-D virus strain (shown in Table 1). This in agreement with Swayne et al.,11 who stated that the more similarity between the AI strains the more reduction in viral shedding.
Yuk et al.,15 showed that while commercial clade 2.3.2 H5 vaccines protected chickens against the HPAI H5N8-D virus challenge, they failed to prevent shedding. Also, Kandeil et al.5) found that although the protection percentage of some commercial H5 vaccines was greater than 90% against the H5N8 strain belonging to clade 2.3.4.4b, with viral reductions in shedding greater than 103 EID50 considered acceptable for any good quality vaccine, other factors can reduce the efficacy of a good quality vaccine, such as uncontrolled field conditions or inadequate biosecurity measures.16
On the other side, it was noticed that despite being the locally prepared inactivated AIV as loc1-H5N1+H5N8 and loc2-H5N8 formulated from clade 2.3.4.4b viral strains, the same as the circulating challenge viruses, provided protection percent of 100% and 90%, respectively. This is in agreement with Swayne et al.,17 who explained that not only the genetic and antigenic match of vaccine strains with circulating HPAI viruses influences vaccine efficacy; other factors, such as manufacturing procedures, adjuvant, antigen content, vaccine dose and administration factors, affect vaccine efficacy, therefore, it is essential to conduct vaccine development studies to improve the percentage of protection and prevent viral shedding against local HPAI H5 viruses in Egypt.14
Conclusions
Vaccines with homologous and heterologous seed virus showed variable degrees of accepted protection percentage ranged from 90% to 100%, thus it was concluded that not only the genetic and antigenic match of the vaccinal strains with the circulating HPAI viruses influences vaccinal efficiency; other factors such as manufacturing procedures, adjuvant, antigen content, vaccine dose and administration factors could affect vaccine efficacy, allowing greater chances of being more immunogenic and effective against different HPAI viruses in endemic regions, therefore, it is essential to conduct further vaccine development studies aimed at improving the protection and prevention of viral shedding against local HPAI H5 viruses in Egypt.