Meu SciELO
Serviços Personalizados
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Nucleus
versão On-line ISSN 2075-5635
Nucleus no.60 Ciudad de La Habana jul.-dez. 2016
CIENCIAS NUCLEARES
Molecular imaging of cancer microenvironment
Imagen molecular del microentorno del cáncer
Alberto Signore, Filippo Galli, Sveva Auletta, Eleonora Briganti and Chiara Lauri
Nuclear Medicine Unit, Department of Medical-Surgical Sciences and of Translational Medicine, Faculty of Medicine and Psychology, “Sapienza” University of Rome, Italy
alberto.signore@uniroma1.it
ABSTRACT
In the last decades, researchers have been focusing on cancer cells looking for novel targets, however, tumours grow in a host environment that either contribute to or inhibit tumour expansion and metastatization. Several efforts have been focused on cancer microenvironment for diagnostic and therapeutic purposes. Nuclear medicine can contribute to understand the complexity and role of tumour microenvironment by imaging several of its components (chemokine receptors, immune cells, stromal antigens, vascular factors, etc). In a tumour, each microenvironment component offers many potential targets for several drugs or radiopharmaceuticals. Cancer may be studied using different strategies from different viewpoints: imaging tumour markers or differentiating markers for diagnostic purposes in order to plan personalized therapies (receptor agonists or superagonists); imaging tumour stroma and vascularization to monitor cell adhesion, metastases, angiogenesis and hypoxia; imaging the host response of cancer cells to monitor efficacy of immunotherapeutic strategies.
Key words: lymphocytes, images, molecules, lymphokines, metastases, diagnosis, therapy, endothelium, growth.
RESUMEN
En las últimas décadas los investigadores han centrado su atención en la observación de las células cancerosas, en búsqueda de nuevos sitios blanco. Sin embargo, el crecimiento del tumor se produce en un entorno que, o inhibe, o contribuye a la expansión del tumor y su metástasis. Varios esfuerzos han estado enfocados al estudio del microentorno del cáncer, con propósitos diagnósticos o terapéuticos. La Medicina Nuclear puede contribuir a la comprensión de la complejidad y del papel que juega el microentorno del tumor, mediante la obtención de las imágenes de varios de sus componentes (receptores de quimioquinas, células inmunes, antígenos del estroma, factores vasculares, etc.). En un tumor, cada componente del microentorno ofrece muchos blancos potenciales para varias drogas o radiofármacos. El cáncer puede ser estudiado mediante diferentes estrategias y enfoques: mediante la imagen de marcadores tumorales, o la diferenciación de estos, con propósitos diagnósticos a fin de planificar terapias personalizadas (receptores agonistas o superagonistas); mediante la imagen del estroma del tumor y la vascularización, para monitorear la adhesión celular, la metástasis, la angiogénesis y la hipoxia; mediante la imagen de la respuesta del huésped de las células cancerosas, con el objetivo de monitorear la eficacia de las estrategias inmunoterapéuticas.
Palabras claves: linfocitos, imágenes, moléculas, linfocinas, metástasis, diagnóstico, terapia, endotelio, crecimiento.
INTRODUCTION
The concept of “microenvironment” has recently gained an important role and it has become the object of several speculations in the last decades in the optic of programming personalized therapies. Tumour growth requires a complex bidirectional interaction between host and cancer cells. As a “parasitic interaction” the cancer cannot exist without the host that provide substances, cytokines, hormones and growth factors that allow malignant cells to take root and spread [1]. The immune surveillance that maintains tissue integrity has a pivotal role in cancer development. Given these premises, the term “immuno-oncology” has been coined and research has been focusing on the concept of cancer microenvironment to clearly understand the underlying mechanisms and to discover novel targets for tailored therapies. In this scenario, nuclear medicine can contribute to clarify the complexity and role of the tumour microenvironment by imaging its components (chemokine receptors, immune cells, stromal antigens, vascular factors, etc). As demonstrated by the presence of infammatory cells in biopsies of many cancers, chronic inflammationis known to have a great importance in oncogenesis providing several triggers that increase the risk of cancers [2, 3]. The hallmarks of cancer related inflammationinclude, of course, cellular and non-cellular elements that compose its microenvironment. This distinction is merely a way to simplify the complex interactions existing between cells and soluble factors produced by cells that exert a paracrine effect on themselves or infuence other distant cells. In non-cellular groups we may include growth or inhibiting factors, cytokines that establish a hormonal and biochemical connection between host and cancer. Among the cellular components we should mention fbroblasts, immune cells, stromal cells and endothelial cells that play a pivotal role in promoting neo-angiogenes, thus leading to cancer progression and metastatization [5-8].
During metastatization, different malignant cells are selected in order to escape from tumour surveillance mechanisms, to survive in blood stream and to take roots at distant sites compromising the prognosis of the patient. This process is also the result of complex interaction between malignant clones and host response [1, 2]. One of the main factors involved in lympho-angiogenesis is vascular endothelial growth factor A (VEGF-A), which is mainly produced by endothelial cells but also by mesenchimal cells and fiboblasts. VEGF acts through the interaction with specificreceptors expressed on the surface of endothelial cells (VEGFR1 and VEGFR2) [9, 10] promoting the formation of new vessels and hindering the correct diffusion of antitumoural drugs [11]. These premises laid the foundation for the development of new targeted drugs like Bevacizumab, an anti-VEGF antibody that prevent VEGF binding to its receptors, thus blocking the synthesis of new vascular and lymphatic vessels. Other drugs, like anti tyrosine kinase inhibitors (TKIs) are able to interfere with VEGFRs signalling. Neoangiogenesis is also indirectly stimulated by the hypoxia inducible factor 1![]() (HIF-1
(HIF-1![]() ) produced in response to hypoxia. Fibroblasts play an important role among the cellular components of cancer microenvironment, since they are able to synthetize different extracellular matrixes through the stimulation of tumour growth factor-β(TGF-β) promoting tumour and vessel growth. Moreover, they can have both stimulatory and inhibitory effects on T-lymphocytes [12]. Other cells that take part in these complex mechanisms are the dendritic cells that have an apoptotic power in cancer cells and produce chemokines that attract for example immune cells natural killer (NK) involved in immune surveillance with high anti-tumour activity [13]. Tumour associated macrophages are important in cancer microenvironment because of their pro or anti-tumour effect. They migrate into cancer and maturate in M1 (anti-tumour effect through the production of pro-inflammatory cytokines for example TNF-
) produced in response to hypoxia. Fibroblasts play an important role among the cellular components of cancer microenvironment, since they are able to synthetize different extracellular matrixes through the stimulation of tumour growth factor-β(TGF-β) promoting tumour and vessel growth. Moreover, they can have both stimulatory and inhibitory effects on T-lymphocytes [12]. Other cells that take part in these complex mechanisms are the dendritic cells that have an apoptotic power in cancer cells and produce chemokines that attract for example immune cells natural killer (NK) involved in immune surveillance with high anti-tumour activity [13]. Tumour associated macrophages are important in cancer microenvironment because of their pro or anti-tumour effect. They migrate into cancer and maturate in M1 (anti-tumour effect through the production of pro-inflammatory cytokines for example TNF-![]() , IL-12) or M2 phenotype (pro-tumour effect through the production of growth factors like VEGF). They also suppress the inflammatory response reducing the effect of antitumour treatments.
, IL-12) or M2 phenotype (pro-tumour effect through the production of growth factors like VEGF). They also suppress the inflammatory response reducing the effect of antitumour treatments.
Other cellular and non-cellular components may be present in a tumour microenvironment with different roles and functions that have not been completely elucidated yet. Therefore, the present review will focus only on those components which represent potential targets for new drugs or radiopharmaceuticals. Indeed, cancer may be studied using different strategies: imaging tumour or differentiating markers for diagnostic purposes in order to plan personalized therapies (receptor agonists or superagonists); imaging tumour stroma and vascularization to monitor cell adhesion, metastases, angiogenesis and hypoxia; imaging the host response to cancer cells to monitor the efficacy of immunotherapeutic strategies. In the next paragraphs we will describe these three main strategies only focusing on part of the complex network of cancer microenvironment.
Cancer cells (over-expression of specific cancer receptors/targets)
Ligands of cancer specificreceptors (receptor agonists or superagonists) may be radiolabeled for diagnostic purposes and in order to plan personalized therapies. A suitable example is provided by thyroid cancer and, in particular, by poorly differentiated (PDTC) or undifferentiated (UDTC) variants that, even if less frequent, are characterized by high death rate (6-10 %) [14]. Well differentiated thyroid tissue expresses sodium/iodide symporter (NIS) on its surface and it is responsible for the uptake of iodine that it is useful for hormonal synthesis. This symporter is also responsible for radio-iodine uptake in the diagnostic and therapeutic fields. Indeed, after surgery the main therapy of differentiated thyroid cancer (DTC) is represented by radio-iodine treatment with ![]() that allows the ablation of thyroid remnant or the treatment of distant or loco-regional metastasis. Du-ring the process of de-differentiation, tumoural cells lose the expression of NIS and, as a result, they will not up-take iodine anymore. This condition is particularly crucial for both a diagnostic and therapeutic point of view becoming refractory to radio-iodine therapy.
that allows the ablation of thyroid remnant or the treatment of distant or loco-regional metastasis. Du-ring the process of de-differentiation, tumoural cells lose the expression of NIS and, as a result, they will not up-take iodine anymore. This condition is particularly crucial for both a diagnostic and therapeutic point of view becoming refractory to radio-iodine therapy. ![]() -Fluoro-Deoxy-Glucose (
-Fluoro-Deoxy-Glucose (![]() -FDG) PET/CT still represents the radiopharmaceutical of choice in the follow-up of patients with high serum thyroglobulin (Tg) and negative
-FDG) PET/CT still represents the radiopharmaceutical of choice in the follow-up of patients with high serum thyroglobulin (Tg) and negative ![]() -whole body scan. Its sensitivity for the detection of distant metastases or local recurrences ranges from 63 % to 98 %, whereas the specificityfrom 81 % to 100 % [15-18]. However, in the last years several second generation PET radiopharmaceuticals have been developed as alternatives to
-whole body scan. Its sensitivity for the detection of distant metastases or local recurrences ranges from 63 % to 98 %, whereas the specificityfrom 81 % to 100 % [15-18]. However, in the last years several second generation PET radiopharmaceuticals have been developed as alternatives to ![]() -FDG, aiming to improve the diagnosis and therapeutic chances of poorly or undifferentiated thyroid cancers [19]. For this purpose
-FDG, aiming to improve the diagnosis and therapeutic chances of poorly or undifferentiated thyroid cancers [19]. For this purpose ,
![]() -FLT,
-FLT, ![]() -somatostatin analogues,
-somatostatin analogues, ![]() -MET and other PET tracers have been proposed showing promising results that have to be confirmed in wider cohorts of patients and with longer follow-ups. Despite these improvements, no specific diagnostic tools and therapies are available yet for patients affected by un-differentiated histotypes. Therefore, it would be desirable to exploit the relationship between cancer and host microenvironment that may offer a wide set of targets for molecular imaging and new therapeutic approaches. It could also be crucial to develop new radiopharmaceuticals for early diagnosis of PDTC and UDTC and for therapy decision making. In thyroid cancer, NIS expression is gradually lost, but TSHR is usually retained, even if not functional [20]. Its natural ligand is the TSH, but the endogenous hormone has a relatively low affinity for its receptor. Through scanning mutagenesis, it was possible to synthesise superagonist rhTSH analogues with a 50-fold higher affinity for the TSHR. Such analogues can be radiolabelled to develop promising radiopharmaceuticals to image radio-iodine refractory thyroid cancer metastases. The rhTSH (Thyrogen
-MET and other PET tracers have been proposed showing promising results that have to be confirmed in wider cohorts of patients and with longer follow-ups. Despite these improvements, no specific diagnostic tools and therapies are available yet for patients affected by un-differentiated histotypes. Therefore, it would be desirable to exploit the relationship between cancer and host microenvironment that may offer a wide set of targets for molecular imaging and new therapeutic approaches. It could also be crucial to develop new radiopharmaceuticals for early diagnosis of PDTC and UDTC and for therapy decision making. In thyroid cancer, NIS expression is gradually lost, but TSHR is usually retained, even if not functional [20]. Its natural ligand is the TSH, but the endogenous hormone has a relatively low affinity for its receptor. Through scanning mutagenesis, it was possible to synthesise superagonist rhTSH analogues with a 50-fold higher affinity for the TSHR. Such analogues can be radiolabelled to develop promising radiopharmaceuticals to image radio-iodine refractory thyroid cancer metastases. The rhTSH (Thyrogen![]() ) has been radiola-belled with
) has been radiola-belled with ![]() I [21, 22] or
I [21, 22] or ![]() [23] and have been tested in vitro and in vivo with good results. In particular, the superagonist rhTSH analogue, radiolabelled with
[23] and have been tested in vitro and in vivo with good results. In particular, the superagonist rhTSH analogue, radiolabelled with ![]() by Galli et al. (
by Galli et al. (![]() -HYNIC-TR1401), seems to be a promising tool in both pre-operative staging of PDTC and follow-up, but more studies are needed to confirmpreclinical results [23].
-HYNIC-TR1401), seems to be a promising tool in both pre-operative staging of PDTC and follow-up, but more studies are needed to confirmpreclinical results [23].
Tumour stroma: endothelial cells, VEGF, VEGFR
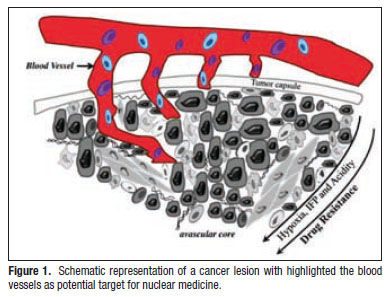
Endothelial cells can be indirectly imaged using radiolabelled VEGF, that as previously described, is an important pro-angiogenic factor (Figure 1).
It is classifiedas a non cellular component of tumoural microenvironment that promotes the growth of endothelial cells deriving from vessels and lymphatics, enhancing vascular permeability and leading to tumour progression and metastatization. These effects are the result of specific pathways activated by the binding of VEGF to its receptors VEGFR1-2-3 and Neuropilin1-2) [9, 10] expressed on both cancer cells and endothelial cells. In the last decades several efforts have been performed in order to develop antiangiogenic therapies that could prevent the binding of VEGF to its receptors (Bevacizumab) or can inhibit tyrosine kinase mediated signalling (Sorafenib). These improvements, together with the increasing attention to personalized therapeutic approach, have led to the development of specific radiopharmaceuticals for the diagnosis and selection of patients eligible to undergo anti-angiogenic treatments in order to predict the response to therapy. Radiolabelled VEGF analogues could be promising radiopharmaceuticals to detect distant metastases of different types of cancers. The most studied variants overexpressed in cancer microenvironment are VEGF165, VEGF206 and VEGF189 mainly located in the extracellular matrix [24]. Another important isoform is VEGF121 that has been bra-diolabeled with ![]() ,
, or
![]() , or with positron-emit-ters to visualize tumour angiogenesis and to monitor therapeutic effects on it [25-31]. Scintigraphic images of
, or with positron-emit-ters to visualize tumour angiogenesis and to monitor therapeutic effects on it [25-31]. Scintigraphic images of ![]() -HYNIC-VEGF in rat models, however, showed high uptake of radiopharmaceutical by several organs (mainly kidneys and liver) resulting in low target/back-ground ratio [32]. A more specificuptake by tumour was observed using
-HYNIC-VEGF in rat models, however, showed high uptake of radiopharmaceutical by several organs (mainly kidneys and liver) resulting in low target/back-ground ratio [32]. A more specificuptake by tumour was observed using ![]() or
or ![]() [33, 34] despite a great up-take from the kidney because of the presence of high concentration of VEFGR in this organ.
[33, 34] despite a great up-take from the kidney because of the presence of high concentration of VEFGR in this organ.
Bevacizumab is a recombinant monoclonal antibody that binds to VEGF-A preventing, in turn, its interactions with VEGFRs. This results in an inhibitory effect on tyrosine kinase mediated pathways, blocking the angiogenesis. Bevacizumab received the approval of FDA for the treatment of metastatic tumours, NSLC and glioblastoma. This monoclonal antibody has been radio-labelled, with good results, with both SPECT and PET radionuclides mainly for colorectal and ovarian cancers [35-39].
Host response to cancer: NK cells, B cells, T cells, NK cells
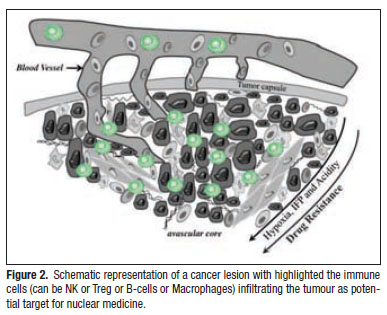
Natural killer cells (NK) are important effectors of immune-surveillance with a marked antitumour activity. Their recruiting in the tumour and their possible use in tumour immunotherapy has been intensively studied (Figure 2). They are a particular subtype of lymphocytes with a ![]() phenotype and they can be divided in two different subsets with different functions. The
phenotype and they can be divided in two different subsets with different functions. The ![]() phenotype shows marked cytotoxic functions and is predominantly present in the peripheral blood and spleen. The
phenotype shows marked cytotoxic functions and is predominantly present in the peripheral blood and spleen. The ![]() subset, present in lymph nodes, has a regulatory function, producing cytokines in response to IL-12, IL-15 or IL-18 stimula-tion [13].
subset, present in lymph nodes, has a regulatory function, producing cytokines in response to IL-12, IL-15 or IL-18 stimula-tion [13].
NK cell activity is regulated by a balance between inhibitory and activating signals. Under certain stimuli, these cells are able to kill target cells without the need of immunization or MHC restriction. ![]() cells are the most potent cytotoxic subtype able to directly kill cancer cells, but can also produce regulatory factors that enhance the host immune response and indirectly limit tumour growth. Cancer cells may present a reduced or altered MHC-I expression that normally allows them to escape T-cell response. These cells can be directly recognized by NKs through a “missing self” mechanism [40]. Similarly, when cancer cells over express certain ligands secondary to DNA damage or stress, they become targets for NK-activating receptors. NK cells directly kill target cells by intracellular cytotoxic granules containing perforin and granzymes that induce either caspase-dependent or caspase-independent apoptosis. The presence of CD16 on the majority of NK surface is also responsible of a direct antitumour effect through antibody-dependent cellular cytotoxicity (ADCC). Other effects of NK on tumours also involve many cytokines, such as IL-12, IL-2, IL-18 or IFN may also enhance the anti-cancer activity of NK cell.
cells are the most potent cytotoxic subtype able to directly kill cancer cells, but can also produce regulatory factors that enhance the host immune response and indirectly limit tumour growth. Cancer cells may present a reduced or altered MHC-I expression that normally allows them to escape T-cell response. These cells can be directly recognized by NKs through a “missing self” mechanism [40]. Similarly, when cancer cells over express certain ligands secondary to DNA damage or stress, they become targets for NK-activating receptors. NK cells directly kill target cells by intracellular cytotoxic granules containing perforin and granzymes that induce either caspase-dependent or caspase-independent apoptosis. The presence of CD16 on the majority of NK surface is also responsible of a direct antitumour effect through antibody-dependent cellular cytotoxicity (ADCC). Other effects of NK on tumours also involve many cytokines, such as IL-12, IL-2, IL-18 or IFN may also enhance the anti-cancer activity of NK cell.
Their indirect antitumour functions are mediated by cytokines (IFN-γ, TNF-![]() and IL-10), chemokines and growth factors that target dendritic cells (DCs), T cells, macrophages and endothelial cells [41]. For example, they can drive T cells polarization toward
and IL-10), chemokines and growth factors that target dendritic cells (DCs), T cells, macrophages and endothelial cells [41]. For example, they can drive T cells polarization toward ![]() cytotoxic phenotype and
cytotoxic phenotype and ![]() toward
toward ![]() to promote CTL diffe-rentiation and are also able to target B cells inducing antitumour antibodies production. Nowadays, many emerging therapies are aimed at increasing the amount of tumour infiltratingNK cells (TINKs). Therefore, imaging of TINKs could allow to image metastases or to follow in vivo the efficacyof newly developed drugs [42]. NK based immunotherapies are undergoing pre-clinical and clinical trials. Tumour xenografts from an anaplas-tic thyroid cancer cell line (ARO), engineered to express IL-12, seem to show a lower proliferation rate than controls ARO cell. In addition, animals seem to have a longer survival enforcing the idea that imaging of TINKs could be useful to evaluate immunotherapy response and for therapy decision making [43]. PET radioisotopes (
to promote CTL diffe-rentiation and are also able to target B cells inducing antitumour antibodies production. Nowadays, many emerging therapies are aimed at increasing the amount of tumour infiltratingNK cells (TINKs). Therefore, imaging of TINKs could allow to image metastases or to follow in vivo the efficacyof newly developed drugs [42]. NK based immunotherapies are undergoing pre-clinical and clinical trials. Tumour xenografts from an anaplas-tic thyroid cancer cell line (ARO), engineered to express IL-12, seem to show a lower proliferation rate than controls ARO cell. In addition, animals seem to have a longer survival enforcing the idea that imaging of TINKs could be useful to evaluate immunotherapy response and for therapy decision making [43]. PET radioisotopes (![]() and
and ![]() ) have been used for imaging NK traffickingin pre-clinical studies. In fibosarcoma models [44] NK cells have been radiolabelled with
) have been used for imaging NK traffickingin pre-clinical studies. In fibosarcoma models [44] NK cells have been radiolabelled with ![]() -methyl iodide to demonstrate that positron emission tomography could be useful to quantify the number of effect or cells, which accumulate into tumours and to determine their biodistribution. Another group injected
-methyl iodide to demonstrate that positron emission tomography could be useful to quantify the number of effect or cells, which accumulate into tumours and to determine their biodistribution. Another group injected ![]() -FDG radiolabeled NKs in HER2/neu positive xenograft models monitoring their traffickingwith autoradiography [45]. For Scintigraphic imaging
-FDG radiolabeled NKs in HER2/neu positive xenograft models monitoring their traffickingwith autoradiography [45]. For Scintigraphic imaging -oxine labelled NK has been studied in metastatic renal carcinoma [46]. Uptake of radiolabelled NK cells, demonstrated by SPECT and also by
![]() -FDG-PET, has been reported in 50% of metastatic lesions however high percentage of circulating
-FDG-PET, has been reported in 50% of metastatic lesions however high percentage of circulating was released by cells. Furthermore,
toxicity negatively influencedNK traffickinginto the tumour. Similar fidings were obtained in melanoma and colorectal cancer [47,48]. The limitations of ex-vivo NK cells radiolabeling can be overcome using anti-CD56 mAb that bind to the immunoglobulin-like adhesion molecule (CD56) expressed on NK surface. The anti-CD56 monoclonal antibody (mAb) was radiolabeled with
![]() and administered in animal models with tumour xenograph of thyroid origin previously injected with human NK [49]. Scintigraphic images performed after 24 hours showed that this radiopharmaceutical could image each tumour with higher T/B ratio than the experiments performed with radiolabelled NK. T/B ratio was also correlated with TINKs infiltration through histological evaluation. In conclusion, the radiolabelled anti-CD56 mAb seems to be a promising tool for non-invasive imaging of NK cell traffickingand follow-up of patients undergoing immunotherapies.
and administered in animal models with tumour xenograph of thyroid origin previously injected with human NK [49]. Scintigraphic images performed after 24 hours showed that this radiopharmaceutical could image each tumour with higher T/B ratio than the experiments performed with radiolabelled NK. T/B ratio was also correlated with TINKs infiltration through histological evaluation. In conclusion, the radiolabelled anti-CD56 mAb seems to be a promising tool for non-invasive imaging of NK cell traffickingand follow-up of patients undergoing immunotherapies.
Tumour-infiltrating B cells (TIL-B)
Tumour-infiltrating B cells (TIL-B) have been studied most extensively in breast and high grade of serious ovarian cancer, where they are present in about 25 % of tumours and comprise up to 40 % of the tumour infiltrating lymphocyte population [50-53]. In tumour microenvironment they are present together with ![]() and
and ![]() T cells and dendritic cells (DCs) [21-23]. One of the most extensively studied targets to image TIL-B is by means CD20 that it is expressed on their surface and that represents a specificmarker of B cells. CD20 is recognised by the mAb Rituximab that is widely used for treatment of non Hodgkin lymphoma (NHL).
T cells and dendritic cells (DCs) [21-23]. One of the most extensively studied targets to image TIL-B is by means CD20 that it is expressed on their surface and that represents a specificmarker of B cells. CD20 is recognised by the mAb Rituximab that is widely used for treatment of non Hodgkin lymphoma (NHL). ![]() radiolabelled rituximab is useful for Scintigraphic imaging of NHL. Planar and SPECT images are able to visualize areas of pathologic uptake of radiopharmaceutical identifying recurrences of the disease [54]. Since
radiolabelled rituximab is useful for Scintigraphic imaging of NHL. Planar and SPECT images are able to visualize areas of pathologic uptake of radiopharmaceutical identifying recurrences of the disease [54]. Since ![]() B cells are involved in several autoimmune diseases;
B cells are involved in several autoimmune diseases; ![]() -rituximab can be also applied for imaging of patient with rheumatoid arthritis, sarcoidosis and Behcet’s disease [55]. From a therapeutic point of view, CD20 is also the target of ibritumomab-tiuxetan (Zevalin
-rituximab can be also applied for imaging of patient with rheumatoid arthritis, sarcoidosis and Behcet’s disease [55]. From a therapeutic point of view, CD20 is also the target of ibritumomab-tiuxetan (Zevalin ![]() ), a
), a ![]() -radiolabelled mAb directed against the same epitope of rituximab, is currently used for treatment of NHL increasing the therapeutic effect of “cold” MoAb [56, 57].
-radiolabelled mAb directed against the same epitope of rituximab, is currently used for treatment of NHL increasing the therapeutic effect of “cold” MoAb [56, 57].
T regulatory cells (Treg)
Naive and activated T cells are able to infiltrate tumours and are subsequently activated through APCs interactions. CD3 is a co-receptor expressed on T cells associated with the TCR. Amongst T lymphocytes, T regulatory cell (Treg), a ![]() T cell subtype, play a pivotal role being recruited in tumours by CCL1 and CCL22 ligands. Therefore, targeting such ligands with specific drug may allow stopping Treg recruitment in tumours and enhancing the host immune response. Treg cells infiltrate tumours producing RANKL (inducing metastases) and suppressing the tumour antigen-specific CTLs [58]. They express CD25, a part of IL-2 receptor that is also expressed by NK cells with lower affinit. IL2 can be used as a surrogate marker for imaging activated T lymphocytes (mainly
T cell subtype, play a pivotal role being recruited in tumours by CCL1 and CCL22 ligands. Therefore, targeting such ligands with specific drug may allow stopping Treg recruitment in tumours and enhancing the host immune response. Treg cells infiltrate tumours producing RANKL (inducing metastases) and suppressing the tumour antigen-specific CTLs [58]. They express CD25, a part of IL-2 receptor that is also expressed by NK cells with lower affinit. IL2 can be used as a surrogate marker for imaging activated T lymphocytes (mainly ![]() ,
, ![]() ).
). ![]() -IL-2 has been extensively studied for several tumours, in particular in melanoma [59], hypernefroma [60], squamous cell carcinomas of head and neck [61] showing optimal biodistribution and dosimetry, high T/B ratio and specific targeting to
-IL-2 has been extensively studied for several tumours, in particular in melanoma [59], hypernefroma [60], squamous cell carcinomas of head and neck [61] showing optimal biodistribution and dosimetry, high T/B ratio and specific targeting to ![]() cells. Recent reports show that the number of
cells. Recent reports show that the number of ![]() cells correlates in-versely with clinical outcomes in several epithelial carcinomas, including ovarian cancer, breast cancer, and hepatocellular carcinoma. In particular, in melanoma
cells correlates in-versely with clinical outcomes in several epithelial carcinomas, including ovarian cancer, breast cancer, and hepatocellular carcinoma. In particular, in melanoma ![]() -IL-2 seems to provide important prognostic information for selection of patients who may benefit from immunotherapy. Therapeutic efficacy of Ipilimumab has already been demonstrated in many studies, however today a surrogate marker of praecox evaluation of response to therapy is still lacking. This aspect could be fundamental in order to select patients that can continue treatment with Ipilimumab and must under go others kinds of therapies.
-IL-2 seems to provide important prognostic information for selection of patients who may benefit from immunotherapy. Therapeutic efficacy of Ipilimumab has already been demonstrated in many studies, however today a surrogate marker of praecox evaluation of response to therapy is still lacking. This aspect could be fundamental in order to select patients that can continue treatment with Ipilimumab and must under go others kinds of therapies. ![]() -IL-2 was used to study 31 patients with cutaneous lesions suspected for melanoma and correlated with histological findings. In 15 of 21 (71 %) melanomas and two of nine (22 %) benign cutaneous lesions, they found uptake of
-IL-2 was used to study 31 patients with cutaneous lesions suspected for melanoma and correlated with histological findings. In 15 of 21 (71 %) melanomas and two of nine (22 %) benign cutaneous lesions, they found uptake of ![]() -IL-2. The calculated T/B ratios correlated significantly with the number of IL-2R-positive TILs [59]. These results suggest a possible role of
-IL-2. The calculated T/B ratios correlated significantly with the number of IL-2R-positive TILs [59]. These results suggest a possible role of ![]() -IL-2 Scintigraphic in the evaluation of response to immunotherapy. This radiopharmaceutical has also been studied in non-oncologic diseases in particular in the fieldof autoimmune-inflammatory diseases for example in diabetes mellitus [62, 63], IBD [64, 65], autoimmune thyroiditis [66].
-IL-2 Scintigraphic in the evaluation of response to immunotherapy. This radiopharmaceutical has also been studied in non-oncologic diseases in particular in the fieldof autoimmune-inflammatory diseases for example in diabetes mellitus [62, 63], IBD [64, 65], autoimmune thyroiditis [66].
CONCLUSION
Tumour microenvironment is the result of very complex interactions between host immune system and cancer cells. Many factors of this network can be potentially targeted by several radiopharmaceuticals in order to image molecular mechanisms that underlie tumour progression, to select patients eligible to peculiar therapies, to valuate the response to treatments. Many efforts have been made in the last years in the fieldof immune-oncology and many others will be performed in next future to improve the knowledge on these aspects aiming at achieving a tailored therapy.
REFERENCES
[1] JOYCE JA & POLLARD JW. Micro environmental regulation of metastasis. Nature Reviews Cancer. 2009; 9(4): 239-252.
[2] BALKWILL F & MANTOVANI A. Inflammation and cancer: back to Virchow? Lancet. 2001; 357(9255): 539-545.
[3] GOUBRAN HA, KOTB RR, STAKIW J, et. al. Regulation of tumour growth and metastasis: the role of tumour microenvironment. Cancer Growth and Metastasis. 2014; 7: 9-18.
[4] MANTOVANI A, ALLAVENA P, SICA A, BALKWILL F. Cancer related-related inflammation. Natue. 2008; 454(7203): 436-444.
[5] ERLER JT, BENNEWITH KL, COX TR, et. al. Hypoxia-induced lysyl oxidise is a critical mediator of bone marrow cell recruitment to form the pre-metastatic niche. Cancer Cell. 2009; 15 (1): 35-44.
[6] ZENG J, XIE K, WU H, et. al. Identification and functional study of cytokines and chemokines involved in tumourgenesis. Combinatorial Chemistry and High Throughput Screening. 2012; 15(3): 276-85.
[7] FOLKMAN J. Role of angiogenesis in tumour growth and metastasis. Semin Oncol. 2002; 29 (6 Suppl 16): 15-8.
[8] MAERTENS L, ERPICUM C, DETRY B, et. al. Bone marrow-derived mesenchymal stem cells drive lymph angiogenesis. PloS One. 2014; 9(9): e106976.
[9] OLSSON AK, DIMBERG A, KREUGER J, CLAESSON-WELSH L. VEGF receptor signalling- in control of vascular function. Nat Rev. Mol Cell Biol. 2006; 7(5): 359-371.
[10] MARU Y, YAMAGUCHI S, SHIBUYA M. Flt-1, a receptor for vascular endothelial growth factor, has trasforming and morphogenic potentials. Oncogene. 1998; 16(20): 2585-95.
[11] GOEL HL, MERCURIO AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013; 13(12): 871-82.
[12] HOOD JD, CHERESH DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002; 2(2): 91-100.
[13] BAUME DM, ROBERTSON MJ, LEVINE H, et. al. Differential responses to interleukin 2 defines functionally distinct subsets of human natural killer cells. Eur J Immunol. 1992; 22(1): 1-6.
[14] PATEL KN, SHAHA AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control. 2006; 13(2): 119-28.
[15] CREACH KM, NUSSENBAUM B, SIEGEL BA, GRIGSBY PW. Thyroid carcinoma uptake of 18F- fluoodeoxyglucose inpatients with elevated serum thyroglobulin and negative 131I scintigraphy. Am J Otolaryngol. 2013 Jan-Feb; 34(1): 51-6.
[16] MIDDENDORP M, SELKINSKI I, HAPPEL C, et. al. Comparison of positron emission tomography with [(18)F]FDG and [(68)Ga]DOTATOC in recurrent differentiated thyroid cancer: preliminary data. Q J Nucl Med Mol Imaging. 2010; 54(1): 76-83.
[17] PALMEDO H, BUCERIUS J, JOE A, et. al. Integrated PET/CT in differentiated thyroid cancer: diagnostic accuracy and impact on patient management. J. Nucl Med. 2006; 47(4): 616-624.
[18] SHAMMAS A, DEGIRMENCY B, MOUNTZ JM, et. al. 18F-FDG PET/CT in patients with suspected recurrent or metastatic well-differentiated thyroid cancer. J. Nucl Med. 2007; 48(2): 221-226.
[19] LAURI C, DI TRAGLIA S, GALLI F, PIZZICHINI P, SIGNORE A. Current status of PET imaging of differentiated thyroid cancer with second generation radiopharmaceuticals. Q J Nucl Med Mol Ima-ging. 2015; 59(1): 105-15.
[20] DURANTE C, PUXEDDU E, FERRETTI E, et. al. BRAF mutations in papillary thyroid carcinoma inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007; 92(7): 2840-3.
[21] CORSETTI F, CHIANELLI M, CORNELISSEN B, et. al. Radioiodinated recombinant human TSH: a novel radiopharmaceutical for thyroid cancer metastases detection. Cancer Biother Radiopharm. 2004; 19(1): 57-63.
[22] SZKUDLINSKI MW, GROSSMANN M, LEITOLF H, WEINTRAUB BD. Human thyroid-stimulating hormone: structure-function analysis. Methods. 2000; 21(1): 67-81.
[23] GALLI F, MANNI I, PIAGGIO G, et. al. (99m)Tc-labeled-rhTSH analogue (TR1401) for imaging poorly differentiated metastatic thyroid cancer. Thyroid. 2014; 24(8): 1297-308.
[24] PHAN HT, JAGER PL, PLUKKER JT, et. al. Comparison of 11C-methionine PET and 18F-fluoodeoxyglucose PET in differentiated thyroid cancer. Nucl Med Commun. 2008; 29(8): 711-6.
[25] PERRI F, PEZZULLO L, CHIOFALO MG, et. al. Targeted therapy: a new hope for thyroid carcinomas. Crit Rev Oncol Hematol. 2014; 94(1): 55-63.
[26] DIJKGRAAF I, BOERMAN OC. Molecular imaging of angiogenesis with SPECT. Eur J Nucl Med Mol Imaging. 2010; 37(Suppl 1): S104-13.
[27] YOSHIMOTO M, KINUYA S, KAWASHIMA A, et. al. Radioiodinated VEGF to image tumour angiogenesis in a LS180 tumour xenograft model. Nucl Med Biol. 2006; 33(8): 963-969.
[28] LI S, PECK-RADOSAVLJEVIC M, KIENAST O, et. al. Imaging gastrointestinal tumours using vascular endothelial growth factor-165 (VEGF165) receptor scintigraphy. .Ann Oncol. 2003; 14(8): 1274-7.
[29] BLANKENBERG FG, BACKER MV, LEVASHOVA Z, et. al. In vivo tumour angiogenesis imaging with site-specific labeled (99m)Tc-HYNIC-VEGF. Eur J Nucl Med Mol Imaging. 2006; 33(7): 841-8.
[30] CHAN C, SANDHU J, GUHA A, et. al. A human transferrin-vascular endothelial growth factor (hnTf-VEGF) fusion protein containing an integrated binding site for (111)In for imaging tumour angiogenesis. J Nucl Med. 2005; 46(10): 1745-52.
[31] LI S, PECK-RADOSAVLJEVIC M, KIENAST O, et. al. Iodine-123-vascular endothelial growth factor-165 (123I-VEGF165). Biodistribution, safety and radiation dosimetry in patients with pancreatic carcinoma. Q J Nucl Med Mol Imaging. 2004; 48(3): 198-206.
[32] LU E, WAGNER WR, SCHELLENBERGER U, et. al. Targeted in vivo labelling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation. 2003; 108(1): 97-103.
[33] HAUBNER R, BEER AJ, WANG H, CHEN X. Positron emission tomography tracers for imaging angiogenesis. Eur J Nucl Med Mol Imaging. 2010; (37 Suppl 1): S86-103. [34] CAI W, CHEN K, MOHAMEDALI KA, et. al. PET of vascular endothelial growth factor receptor expression. J Nucl Med. 2006; 47(12): 2048-56.
[35] NAGENGAST WB, HOOGE MN, van STRATEN EM, et. al. VEGF-SPECT with ¹¹¹In-bevacizumab in stage III/IV melanoma patients. Eur J Cancer. 2011; 47(10): 1595-602.
[36] HOSSEINIMEHR SJ, ORLOVA A, TOLMACHEV V. Preparation and in vitro evaluation of 111In-CHX-A”-DTPA-labeled anti-VEGF monoclonal antibody bevacizumab. Hum Antibodies. 2010; 19(4): 107-11.
[37] PAUDYAL B, PAUDYAL P, ORIUCHI N, et. al. Positron emission tomography imaging and biodistribution of vascular endothelial growth factor with 64Cu-labeled bevacizumab in colorectal cancer xenografts. Cancer Sci. 2011; 102(1): 117-21.
[38] ASHRAFI SA, HOSSEINIMEHR SJ, VARMIRA K, ABEDI SM. Radioimmunotherapy with ¹³¹I-bevacizumab as a specificmolecule for cells with overexpression of the vascular endothelial growth factor. Cancer Biother Radiopharm. 2012; 27(7): 420-5.
[39] GAYKEMA SB, SCHRÖDER CP, VITFELL-RASMUSSEN J, et. al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res. 2014; 20(15): 3945-54.
[40] KÄRRE K, LJUNGGREN HG, PIONTEK G, KIESSLING R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. J Immunol. 2005; 174(11): 6566-9.
[41] SCHOTT M. Immunesurveillance by dendritic cells: potential implication for immunotherapy of endocrine cancers. Endocr Relat Cancer. 2006; 13(3): 779-95.
[42] CHENG M, CHEN Y, XIAO W, et. al. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013; 10(3): 230-52.
[43] SHI Y, PARHAR RS, ZOU M, et. al. Gene therapy of anaplastic thyroid carcinoma with a single-chain interleukin-12 fusion protein. Hum Gene Ther. 2003; 14(18): 1741-51.
[44] MELDER RJ, BROWNELL AL, SHOUP TM, et. al . Imaging of activated natural killer cells in mice by positron emission tomography: preferential uptake in tumours. Cancer Res. 1993; 53(24): 5867-71.
[45] MEIER R, PIERT M, PIONTEK G, et. al. Tracking of [18F]FDG-labeled natural killer cells to HER2/neu-positive tumours. Nucl Med Biol. 2008; 35(5): 579-88.
[46] MELLER B, FROHN C, BRAND JM, et. al. Monitoring of a new approach of immunotherapy with allogenic (111)In-labelled NK cells in patients with renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2004; 31(3): 403-7.
[47] MATERA L, GALETTO A, BELLO M, et. al. In vivo migration of labeled autologous natural killer cells to liver metastases in patients with colon carcinoma. J Transl Med. 2006; 4: 49.
[48] SCHÄFER E, DUMMER R, EILLES C, et. al . Imaging pattern of radiolabeled lymphokine-activated killer cells in patients with metastatic malignant melanoma. Eur J Nucl Med. 1991; 18(2): 106-10.
[49] GALLI F, HISTED S, ARAS O. NK cell imaging by in vitro and in vivo labelling approaches. Q J Nucl Med Mol Imaging. 2014; 58(3): 276-83.
[50] NELSON BH. CD20+ B cells: the other tumour-infiltrating lymphocytes. J Immunol. 2010; 185(9): 4977-82.
[51] CORONELLA-WOOD JA, HERSH EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol. Immunother. 2003; 52(12): 715-738.
[52] CHIN Y, JANSEENS J, VANDEPITTE J, et. al. Phenotypic analysis of tumour-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992; 12(5): 1463-1466.
[53] MARSIGLIANTE S, VISCOSO L, MARRA A, et. al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999; 139(1): 33-41.
[54] GMEINER STOPAR T, FETTICH J, ZVER S, et. al. 99mTc-labelled rituximab, a new non-Hodgkin’s lymphoma imaging agent: first clinical experience. Nucl Med Común. 2008; 29(12): 1059-65.
[55] MALVIYA G, ANZOLA KL, PODESTA E, et. al. (99m)Tc-labeled Rituximab for Imaging B Lymphocyte Infiltration in Inflammatory Autoimmune Disease Patients. Mol Imaging Biol. 2012; 14(5): 637-46.
[56] DAVIS TA, KAMINSKI MS, LEONARD JP, et. al. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res. 2004; 10(23): 7792-7798.
[57] IVANOV A, KRYSOV S, CRAGG MS, ILLIDGE T. Radiation therapy with tositumomab (B1) anti-CD20 monoclonal antibody initiates extracellular signal-regulated kinase/mitogen-activated protein kinase-dependent cell death that overcomes resistance to apoptosis. Clin Cancer Res. 2008; 14(15): 4925-4934.
[58] CURIEL TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007; 117(5): 1167-74.
[59] SIGNORE A, ANNOVAZZI A, BARONE R, et. al. 99mTc-interleukin-2 scintigraphy as a potential tool for evaluating tumour-infiltrating lymphocytes in melanoma lesions: a validation study. J Nucl Med. 2004; 45(10): 1647-52.
[60] RENARD V, STAELENS L, SIGNORE A, et. al. Iodine-123-interleu-kin-2 scintigraphy in metastatic hypernephroma: a pilot study. Q J Nucl Med Mol Imaging. 2007; 51(4): 352-6.
[61] LOOSE D, SIGNORE A, STAELENS L, et. al. (123) I-Interleukin-2 uptake in squamous cell carcinoma of the head and neck carcinoma. Eur J Nucl Med Mol Imaging. 2008; 35(2): 281-6.
[62] SIGNORE A, CAPRIOTTI G, CHIANELLI M, et. al. Detection of insulitis by pancreatic scintigraphy with 99mTc-labeled IL-2 and MRI in patients with LADA (Action LADA 10). Diabetes Care. 2015; 38(4): 652-8.
[63] CHIANELLI M, PARISELLA MG, VISALLI N, et. al. Pancreatic scintigraphy with 99mTc-interleukin-2 at diagnosis of type 1 diabetes and after 1 year of nicotinamide therapy. Diabetes Metab Res Rev. 2008; 24(2): 115-22.
[64] ANNOVAZZI A, BIANCONE L, CAVIGLIA R, et. al. 99mTc-interleukin-2 and (99m)Tc-HMPAO granulocyte scintigraphy in patients with inactive Crohn’s disease. Eur J Nucl Med Mol Imaging. 2003; 30(3): 374-82.
[65] SIGNORE A, CHIANELLI M, ANNOVAZZI A, et. al. Imaging active lymphocytic infiltration in coeliac disease with iodine-123-inter-leukin-2 and the response to diet. Eur J Nucl Med. 2000; 27(1): 18-24.
[66] CHIANELLI M, MATHER SJ, GROSSMAN A, et. al. 99mTc-interleukin-2 scintigraphy in normal subjects and in patients with autoimmune thyroid diseases: a feasibility study. Eur J Nucl Med Mol Imaging. 2008; 35(12): 2286-93.
Recibido: 20 de mayo de 2016
Aprobado: 28 de julio de 2016