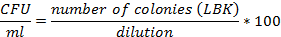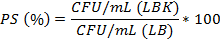Introduction
Human Papilloma viruses (HPV) are a long and complex group of more than 200 different types of viruses belonging to the Parvoviridae family. The viral capsid is composed by 72 capsomeres, which consist of L1 and L2 proteins. The L1 is the major protein with a molecular weight of 55 kDa (Hagensee, Yaegashi, & Galloway, 1993). HPV infection is one of the most common sexually transmitted disease globally. Generally, the infections are clear by the immune system, but sometimes this response fails and the infection persists, causing warts or cancer (McGill, et al., 2020). The high risk oncogenic HPV 16 and 18 genotypes are responsible for around 70% of cervical cancer cases (CCU) worldwide (World Health Organization, 2019), being the HPV16 the more frequent genotype detected in cervical cancers, meanwhile the HPV18 is most often associated with genital carcinoma. This is the third kind of cancer in prevalence on women globally, and the second type in the less developed countries (ICO/IARC HPV Information Centre, 2019a). In Cuba, it is the fourth variety of cancer in incidence and mortality in women of all ages, and the second on females between 15 to 44 years old (ICO/IARC HPV Information Centre, 2019b). Due to the high burden of HPV-related diseases, the World Health Organization recommends to include preventive vaccines in the National Immunization Programs.
Three licensed preventive HPV vaccines are currently available on the market: Gardasil, Gardasil-9 and Cervarix (Asociación Americana contra el cáncer, 2018). The three protect against infections by the HPV 16 and 18 types; Gardasil further protects against the HPV 6 and 11 virions, responsible for 90% of genital wart cases (Koutsky, Ault, Wheeler, & al., 2002), and Gardasil 9 also prevents infections with high risk oncogenic HPV 31, 33, 45, 52 and 58 (Chatterjee, 2014). These HPV vaccines are based on L1-assembled virus-like particles (VLPs), potent immunogens that induce high neutralizing antibody titers, protecting against targeted HPV infections (Lowy, 2016). These VLPs are obtained in eukaryotic hosts with high production costs; hence these vaccines have high prices, which limit their deployment in low-income countries where cervical cancer results in a high mortality (Kim, Lee, & Kim, 2010). The need for a cheaper new prophylactic vaccine candidate against HPV 16 and 18 has guided the work at our laboratory in the last years, using Escherichia coli as expression system. This alternative expression host is not only more economical, but it has allowed developing a new bivalent HPV-vaccine by Chinese researchers (Quiao, et al., 2020).
Previously, a full-length HPV18-L1 gene isolated from a Cuban patient´s sample was cloned in E. coli (Pimienta, et al., 2018) and subsequently it was subcloned into the pET28a expression vector fused to the six-His-tag-coding sequence at the 5´-end of the gen (HPV18-His-tag-L1) to facilitate future purification of the protein. Comparison of the HPV18-His-tag-L1 protein production by E. coli BL21 (DE3), Origami (DE3) and Rosetta (DE3) pLysS under IPTG induction, found that L1 protein was produced in insoluble form and at higher amounts by E. coli BL21 (DE-3) (Pimienta, et al., 2019). Alternative inducing conditions such as low-IPTG concentrations or autoinduction conditions have conducted to soluble protein production in E. coli, allowing to obtain higher levels of active folded proteins (Pimienta, et al., 2018; Faust, Stand, & Weuster‐Botz, 2015). On the other hand, preparation of a working cell bank (WCB) is considered the first important step to establish a robust purification process (Sobolewska-Ruta & Zaleski, 2019). The aims of this study were to prepare a WCB of E. coli BL21 (DE3) harboring a HPV18-His-Tag-L1-pET28a derivative expression plasmid, as well as to evaluate production and solubility of HPV18-His-tag-L1 protein by the bank under isopropyl-β-D-1-thiogalactopyranoside (IPTG) induction and on auto-inducing conditions, as the first step to tackle L1 purification.
Methodology
Preparation of the E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB. The master cell bank (MCB) of E. coli BL21 (DE3) [pET28a-HPV18L1tag] (Pimienta, et al., 2019) was inoculated into 5 mL of ZYM-505 broth (Studier, 2005) supplemented with 100 μg/mL kanamycin and incubated overnight at 26-28ºC and 220 revmin-1. Then, the cells were inoculated into 400 mL of fresh ZYM-505 medium at an optical density at 600 nm (OD600) of 0.04, and incubated under the same conditions until the OD600 reached 0.8-0.9. After, cells were harvested by centrifugation at 3500 revmin-1 at 4ºC and the cellular pellet was suspended at a final OD600=20 with Luria Bertani (LB) broth (10g/L tryptone, 5g/L yeast extract and 10g/L NaCl) supplemented with glycerol at 20% (w/v). Aliquots of 1 mL were dispensed and conserved at -70oC.
Evaluation of the E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB. For assessing cellular viability and plasmid stability, three aliquots of the WCB were serially diluted in 10-fold steps up to 10-9. Aliquots (10μL) in triplicate from 10-5 to 10-9 dilutions were applied in parallel on LB-agar at 1.5% agar (w/v) and LB-agar-kanamycin (100 μg/mL, LBK) plates and incubated at 30ºC overnight. The isolated colonies were counted and the cellular viability was estimated as the colony forming units (CFU) grown on LBK plates, using the following formula:
Plasmid stability (PS, %) was estimated according to the formula:
Assessment of HPV18-His-Tag-L1 production by E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB at a small scale. A WCB aliquot was inoculated into 5mL of Terrific Broth (TB) (12g/L tryptone, 24g/L yeast extract, 31 g/L KH2PO4, 12,54 g/L K2HPO4 and 0,4 % (w/v) glycerol) with kanamycin (100 μg/mL) and incubated overnight at 220 revmin-1 and 26-28ºC. Then, the culture was diluted 1/100 into 10 mL of fresh TB at 250mL shake flask scale, and grown at 37°C and 250 revmin-1 until the OD600 reached 1.2 - 1.6 units, when the protein production was induced with IPTG (60 µmolL-1) for 5 h at 26-28°C. Aliquots of 1 mL were centrifuged and the cellular pellet was suspended on cold 1X Phosphate-Buffered Saline (PBS) at a final OD600=6.5. Protein production was analyzed by 10% (w/v) sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, followed by staining with Coomassie brilliant blue or Western blot analysis using anti-His antibody (H8) (Merck) to detect the HPV18- His-tag-L1 protein and a rabbit anti-Mouse IgG peroxidase-conjugated antibody as secondary antibody. The % of L1 protein regarding total proteins was estimated by densitometric analyses using the GelAnalizer program. Statistical analyses were done with Graph Pad Prism 5.01, using t student test.
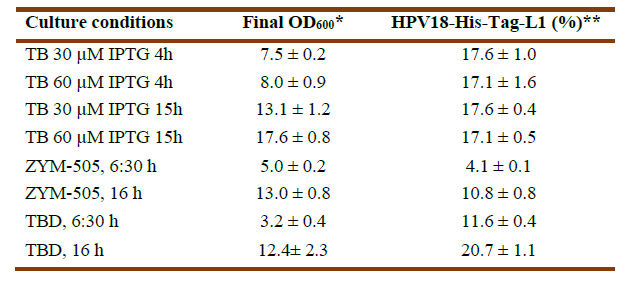
Evaluation of HPV18-His-Tag-L1 production by E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB at 2L-shake flask scale. HPV18-His-Tag-L1 synthesis by the WCB at a 2L-shake flask scale (200 mL of medium) was evaluated under TB, ZYM-505 (with 0.2% (w/v) α-lactose) and TB Diederich (TBD) (TB with 1% (w/v) glycerol and 0.2% (w/v) α-lactose) media (Diederichs, et al., 2014). The bank was directly inoculated on the different media at an initial OD600=0.1. In the case of the auto-induction medium, cultures were incubated at 26-28ºC and 250 revmin-1 for 6:30 and 16h. In the case of IPTG-induction on TB, two inducer concentrations were used (30 and 60 μmolL-1) for 4h and 15 h. The L1 protein production was evaluated by SDS-PAGE and the percent regarding total proteins it represented were estimated by densitometric analyses using the GelAnalizer program. For statistical analyses, the Graph Pad Prism 5.01, using Tukey´s multiple comparison test on One-Way ANOVA, was used.
Assessment of HPV18-His-Tag-L1 protein´s solubility. E. coli BL21 (DE3) [pET28a-HPV18L1tag] cultures were harvested by centrifugation at 5000 revmin-1 for 10 min at 4°C and the cellular pellet was washed twice with cold 1X PBS. Cells were disrupted by an EmulsiFlex-C3 High Pressure Homogenizer, using 10 mL of lysis buffer (Hepes 20 mmolL-1, pH 8, 300 mmolL-1 NaCl, 1 mmolL-1 EDTA, 1mmolL-1 phenylmethylsulfonyl fluoride (PMSF), 1mmolL-1 Dithiothreitol (DTT) and 1% (v/v) Triton X-100) per gram of cells (wet weight). Soluble and insoluble fractions were analyzed by 10 % SDS-PAGE.
Results and Discussion
The cell bank is the most important starting material of a biotechnological process. Production of recombinant proteins should be based on well-defined MCB and WCB. All cell banks should be characterized for relevant phenotypic and genotypic markers, which could include evaluating the presence of the expression construct and production of the recombinant protein. In the present study, a WCB of E. coli BL21 (DE3) [pET28a-HPV18L1tag] was prepared and the HPV18-His-Tag-L1 protein production was evaluated under IPTG-induction and auto-induction medium, as these approaches have been used successfully to improve production and solubility of other recombinant proteins (Pimienta, et al., 2019; Studier, 2005; Seo, et al., 2009).
Characterization of E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB
The E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB had 3 ± 0.5 ×109 viable cells/mL and 100% of the tested colonies were kanamycin-resistant, indicating they harbored the expression plasmid. These values allow to use the WCB for producing the HPV18-His-Tag-L1 protein for developing a purification protocol.
Synthesis of HPV18-His-tag-L1 protein by E. coli BL21 (DE3) [pET28a-HPV18L1tag] MCB and WCB was compared under IPTG-induction (60 μmolL-1) on TB for 5h and analyzed by SDS-PAGE (Fig. 1A). Densitometric analysis of the gel showed that HPV18-His-tag-L1 protein was produced at similar levels by the working and the master-cell banks (Fig. 1B), without statistically significant differences between them (t student, p=0.1705).
analyzed by SDS-PAGE (Fig. 1A). Densitometric analysis of the gel showed that HPV18-His-tag-L1 protein was produced at similar levels by the working and the master-cell banks (Fig. 1B), without statistically significant differences between them (t student, p=0.1705).
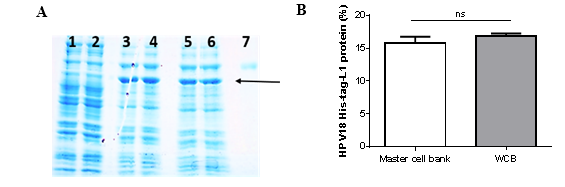
Fig. 1 HPV18-His-Tag-L1 production by E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB. Cultures were grown on Terrific Broth until exponential phase and induced with IPTG (60 µmolL -1 ) for 5h. A: 10 % SDS-PAGE of whole cell lysate of E. coli BL21 (DE3): lines 1-2, without plasmid; lines 3-4, pET28a-HPV18L1tag-master cell bank and lines 5-6, pET28a-HPV18L1tag-WCB; Line 7, bovine serum albumin (molecular marker, 66.3 kDa). The arrow indicates L1 protein. B: Densitometric analysis of gel. The columns represent the means ± SD (N=4). The means were not significantly different (t student, p=0.1705).
Production of HPV18-His-tag-L1 by E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB under IPTG- induction and auto-induction conditions at 2L-shake flask scale
HPV18-His-tag-L1 protein production by E. coli BL21 (DE3) [pET28a-HPV18L1tag] induced by IPTG (100 µmolL-1) was previously reported (Pimienta, et al., 2018). However, determining the minimal IPTG concentration required for inducing L1 synthesis at a higher scale was required. The production of HPV18-His-tag-L1 by E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB in response to different IPTG concentrations (from 15 µmolL-1 to 4 mmolL-1) was evaluated by Western blot (Fig. 2A). The amount of L1 protein remains constant for IPTG concentrations higher than 60 µmolL-1 (Fig. 2B), without significant differences among 4, 2, 0.5, 0.25, 0.125 and 0.06 mmolL-1 concentrationsof IPTG (Fig. 2B; One Way ANOVA, Tukey´s multiple comparison test, p˃0.05). High IPTG concentrations is reported to affect growth of recombinant E. coli and protein´s production and solubility, which could be mediated by its toxic effect (Leites, et al., 2014). Considering these results, IPTG concentrations of 30 and 60 µmolL-1 were selected for inducing L1 production at 2L-shake flask scale.
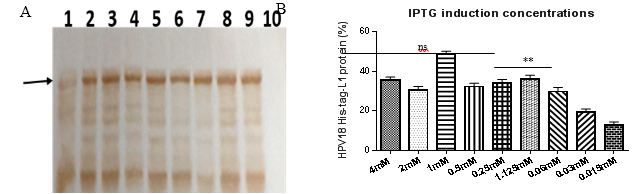
Fig 2 Production of HPV18-His-Tag-L1 by E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB induced with different IPTG concentrations. A: Western blotting of whole cell lysates of E. coli BL21 (DE3) [pET28a-HPV18L1tag] induced with IPTG at: lines 1, 2, 3, 4, 5, 6, 7, 8 and 9: 0.015, 0.03, 0.06, 0.125, 0.25, 0.5, 1.0, 2.0 and 4.0 mmolL -1 , respectively. Line 10, E. coli BL21 (DE3) [pET28a], 4 mmolL -1 IPTG. B: Densitometric analysis of the blot. Statistical analysis was performed on Graph Pad Prism 5.01 program. The columns represent the means ± SD (N= 2 per group). Significant differences among 4, 2, 0.5, 0.25, 0.125 and 0.06 mmolL -1 IPTG were not detected (One-Way ANOVA, Tukey’s multiple comparison test, *p<0.05, **p<0.01). The assay was repeated twice, with similar results.
Synthesis of HPV18-His-tag-L1 by E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB was induced with IPTG (30 and 60 μmolL-1) for 4h and 15h on TB, as well as on ZYM-505 and TBD auto-induction media for 6:30h and 16h (Fig. 3A). Densitometric analyses showed that HPV18-His-tag-L1 expression was maximal on IPTG-induced TB from 4h (Fig. 3B); however, an additional incubation for 16h allowed to reach also high cell densities (Table 1), with the subsequent increase in biomass productivity. In addition, it was also found that 30 μmolL-1 of IPTG was enough to promote L1 production, since the amount of L1 protein was no statistically different at 30 and 60 μmolL-1 (Fig. 3B), which is a very important result when producing the protein to a higher scale.
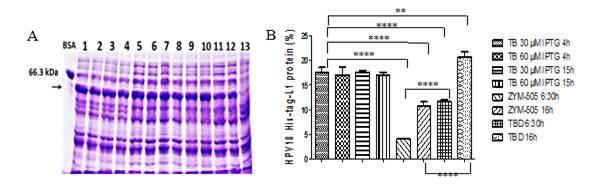
Fig. 3 HPV18-His-Tag-L1 production by E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB under IPTG- and auto-induction conditions. A: Representative 10% SDS-PAGE of whole cell lysates: induced for 15h on TB with IPTG at 30 µmolL -1 (lines 1 and 2) and 60 µmolL -1 (lines 3 and 4); for 4h on TB with IPTG at 30 (lines 5 and 6) and 60 µmolL -1 (lines 7 and 8); and autoinduced on TBD for 6:30 h (lines 9-10) and 16 h (lines 11-12). Line 13, E. coli BL21 (DE3) without plasmid, cultured on TB for 15h. The arrow indicates L1 protein. BSA, bovine serum albumin. B: Densitometric analysis of the gel. Statistical analyses were performed by using Graph Pad Prism 5.01 program. The columns represent the means ± SD. It was applied an One-Way ANOVA, Tukey’s multiple comparison test (*p<0.05, **p<0.01, ***p<0,001, ****p<0.0001; N= 2 per group). The assay was replicated three times, with similar results.
In the case of the evaluated auto-induction media, HPV18-His-tag-L1 was produced at statistically significant higher amounts on TBD than on ZYM-505 for the two evaluated times (Fig. 3B). Incubation for 16h allowed to reach a higher final cellular density and L1 protein´s accumulation (Table 1), indicating that L1 protein was not degraded and was synthesized more slowly under these conditions in comparison with the conventional IPTG-induction.
Cellular localization of HPV18-His-tag-L1 protein under the evaluated induction conditions
Cellular localization of HPV18-His-Tag-L1 protein was evaluated on cells grown on TBD for 16 h and on cells induced with 30 µmolL-1 IPTG for 4h, considering the highest protein levels and the shorter incubation time, as well as the lower inducer concentration. In both conditions, the L1 protein was mainly found in the insoluble fractions (Fig. 4, lines 4 and 6). These results are in agreement with the insoluble production of this protein by E. coli SHuffle T7 (Pimienta, et al., 2018) and HPV16 L1 expressed by E. coli (Zhang, et al., 1998). The effects of lower temperatures on the solubility of the HPV18 L1 protein should be studied in further experiments. The purification of HPV18-His-tag-L1 from the insoluble fraction of both culture conditions is in progress, using Immobilized-Metal Affinity chromatography, as reported for HPV16 L1-His-Tag protein by Brito et al, 2020. The structural quality of the HPV18 L1 protein produced under both culture conditions could be different; as it has been shown that the physicochemical characteristics of inclusion bodies change in response to different culture conditions in E. coli (Castellanos, et al., 2014)

Fig. 4 HPV18-His-Tag-L1 solubility in E. coli BL21 (DE3) [pET28a-HPV18L1tag] WCB grown under IPTG- and auto-induction conditions. 10% SDS-PAGE of cell lysates of E. coli BL21 (DE3) [pET28a-HPV18L1tag] bank cultured under IPTG- and auto-induction conditions. Line 1 and 2, total fraction on TBD and IPTG induction, respectively; line 3 and 4, soluble fraction and insoluble fraction on TBD, respectively, and line 5 and 6, soluble fraction and insoluble fraction on IPTG induction, respectively.
Conclusions
In the present study, a working cell bank of E. coli BL21 (DE·) [pET28a-HPV18L1tag] was prepared and characterized. The production of HPV18-His-Tag-L1 protein by the bank was induced on TB medium under low IPTG concentration (30 and 60 µmolL-1) and on ZYM-505 and TBD auto-inducing media. Only under IPTG-induction and TBD auto-induction for 16h was produced the recombinant protein about nearly 20% of total proteins. Furthermore, the produced protein was mostly found in the insoluble fraction, requiring denaturing conditions for its purification, in agreement to previous reports for the HPV L1 production in E. coli. The available WCB will allow maintaining the constant quality and homogeneity of the initial inoculum during L1 production to develop a robust purification.