INTRODUCTION
Psidium is a genus of at least 60 species and perhaps as many as 100, ranging from Mexico and the Caribbean to Argentina and Uruguay (Landrum, 2017). A few species have been introduced as cultivated plants in the Old World and Pacific Island tropics and subtropics, and some are weedy invasives (Global Invasive Species Database, 2020). Taxonomic studies of Psidium have been numerous in the last few years with several new species being described (Landrum, 2017).
Recently, Psidium acutangulum DC. as it has traditionally been recognized east of the Andes, was divided into two species: P. acutangulum and P. acidum (DC.) Landrum; and some of the populations of Psidium from western Ecuador on the Pacific slope that have previously been identified as P. acutangulum, should be recognized as a new species (Landrum, 2016).
P. acidum is native to tropical South America and it is a large shrub or small tree, commonly known in some American countries as “guayaba de monte”, “sacha guayaba”, “guayaba agria” and “guayaba ácida”. The fruit is a smooth spherical berry (6-7 cm across), glabrous, green turning to yellow when ripe, with persistent calyx remnants at the apical end and yellowish-white, very acidic but strongly flavored pulp containing a few hard and triangular seeds (Trujillo et al., 2018). The fruit is consumed fresh, but commonly it is used to prepare juices and sorbets.
No work has yet been published in the literature to characterize the volatile compounds of sour guava fruit. Therefore, the aim of the present study was to analyze its volatile compounds and to investigate the aroma-active compounds by headspace-solid phase microextraction (HS-SPME).
Materials and Methods
Samples and chemicals
Sour guava fruits were grown using standard agricultural practices in the University of Ciego de Avila, Cuba. Fruits were harvested at fully yellow maturity stage and immediately transported to the laboratory. Fruits were cut, and the peel and seeds were removed. The pulp was homogenized using a commercial blender. Plant materials were deposited in the Julián Acuña Galé herbarium (Ignacio Agramonte University, HIPC-Thiers, 2018; voucher HPC-12030).
Reference compounds were purchased from Sigma-Aldrich (Steinheim, Germany) and Merck (Darmstad, Germany), and some others were generously given by Robertet (Grasse, France). A normal paraffin solution (C5-C24) was supplied by Sigma-Aldrich. Sodium chloride was provided by Merck (Darmstadt, Germany).
Standard chemical analysis
Total soluble solids, total acidity (as anhydrous citric acid), pH and ascorbic acid content were assayed in fruit samples according to official methods (AOAC, 2019).
Headspace solid-phase microextraction analysis
Volatile compounds from the fresh fruit homogenate headspace were extracted using four SPME fiber coatings: 100 (m PDMS, 65 (m PDMS/DVB, 50/30 (m DVB/CAR/PDMS, and 85 (m CAR/PDMS (Supelco, Park, Bellefonte, Pa.). All the fibers were conditioned before use and cleaned between analyses by inserting them into the GC injector. HS-SPME extraction was performed at 40 oC on 3 g of pulp, 3 mL distilled water and 1 g NaCl contained in a 15-mL vial sealed with a PTFE-lined screw cap. A pre-extraction time of 10 min, and an extraction time of 30 min under magnetic stirring at 600 min-1 were applied. The sampling conditions were chosen after preliminary GC-FID analyses and were like those reported in other studies (Pino & Febles, 2013; Pino & Bent 2013; Pino, 2014).
GC-FID and GC-MS analysis
A Hewlett-Packard 6890N series II (Agilent, Santa Clara, CA, USA) gas chromatograph equipped with a 30 m x 0.25 mm x 0.25 μm DB-5ms (J & W Scientific, Folsom, CA) and a flame ionization detector (FID) was used. Oven temperature was held at 50 °C for 2 min and then raised to 280 °C at 4 °C/min and held for 10 min. Carrier gas (hydrogen) flow rate was 1 mL/min. Injector and detector were set at 250 oC. Injection was in splitless mode (2 min) and with a recommended liner of 0.75 mm. Linear retention indexes were calculated using a mixture of normal paraffins. quantitative determinations were based on the normalization method assuming similar calibration factors for all the compounds.
GC-MS analysis was performed on a QP-2010 Ultra (Shimadzu, Japan) with the same capillary column and chromatographic parameters as for the GC-FID. Helium carrier gas flow rate was 1 mL/min. MS data were recorded in a mass range 35-350 u, with electron energy of 70 eV and ion source and connecting parts temperature, 250 oC. The identification of compounds was achieved by comparison of their linear retention indexes and mass spectra with those shown by reference standards when they were available. In other cases, comparison was made with those in commercial databases (NIST 05, NBS 75 k, Wiley 6 and Adams 2001).
HS-SPME direct gas chromatography-olfactometry (GC-O)
A Hewlett-Packard 6890N series II gas chromatograph equipped with a FID and a sniffing port joined to the injector by a short stainless-steel capillary (25 cm x 0.4 mm i.d.). The flow rate of the carrier gas (H2) was 25 mL/min, and the oven temperature was kept at 250 oC. The three SPME extracts were introduced in successive sequences into the GC port at 250 oC. Because no chromatographic separation was carried out by the short capillary, volatile compounds arrived simultaneously at the sniffing port. Here, for each SPME extract, a trained panel of three sniffers perceived and evaluated the resulting global odor. Fibers were kept in the GC inlet until the end of the sensorial stimulus.
Sensory analysis sessions were performed only after a suitable training: sniffers were first familiarized with fresh fruit and asked to agree on a common list of descriptors. A similarity test was performed in triplicate on the same homogenate. Sniffers were asked to smell the reference pulp (2 mL) contained in a plastic cup sealed with a pierced cap at 25 °C. They had to memorize the odor and then describe it using the descriptors list. Then they evaluated with the direct GC-O device the different extracts, rating their similarity to the reference using a 10-cm scale ranging from 0 (close to the reference) to 10 (far from the reference). Sniffers had to smell the reference before each sample evaluation. All analyses were replicated three times.
Gas chromatography-olfactometry of HS-SPME extract
The odor-active compounds of DVB/CAR/PDMS SPME extracts were analyzed by GC-O on the above mentioned GC-FID equipped with the same capillary column mentioned above, connected via two fused silica capillaries (25 cm x 0.25 mm i.d.) to a FID and a sniffing port described earlier (Pino & Febles, 2013). After sampling, the SPME fiber was desorbed for 5 min into the injection port at 250 °C. Operating conditions were the same as described before for GC-FID. The GC effluent was split 1:1 between the FID and the sniffing port (both at 250 °C). Injection volume was 1 μL. For each odor stimulus, the three sniffers recorded the detection time and odor description. GC-O frequency analysis was performed following the methodology described earlier (Chaintreau, 2001). Detected odors (quality and retention times) were marked in the chromatogram. Each sample was sniffed in triplicate by each sniffer. Zones of the chromatogram which were detected with the same descriptor, at least three times, were considered as odor zones.
Results and Discussion
Four fibers, coated with 100 (m PDMS, 65 (m PDMS/DVB, 50/30 (m DVB/CAR/PDMS and 85 (m CAR/PDMS, were assessed for the isolation of the volatile compounds. The similarity scaling obtained for the four SPME global odors with respect to the reference sample were DVB/CAR/PDMS (1.0 ± 0.2), PDMS/DVB (3.5 ± 0.3), CAR/PDMS (7.2 ± 0.3) and PDMS (8.2 ± 0.5) and. The reason might be that the distribution coefficient of three phase between coating and sample exist in the process of extraction. Thus, the 50/30 μm DVB/CAR/PDMS fiber was selected in this study.
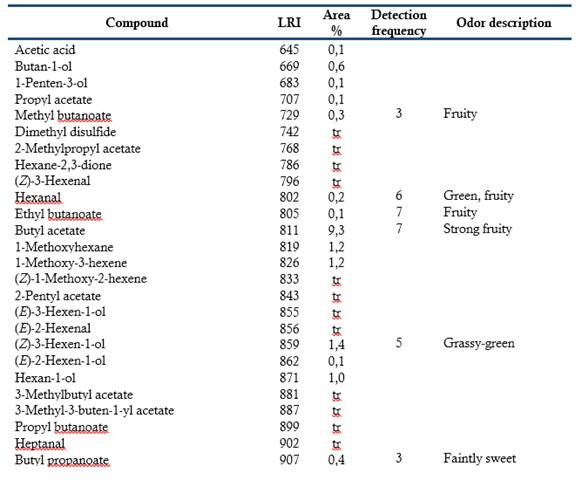
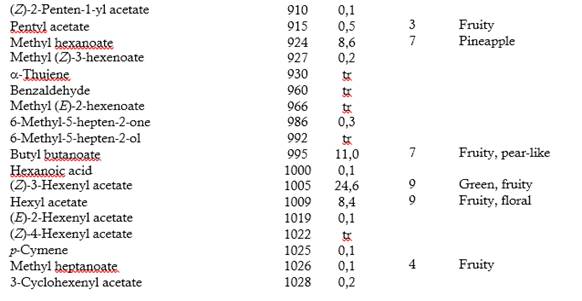
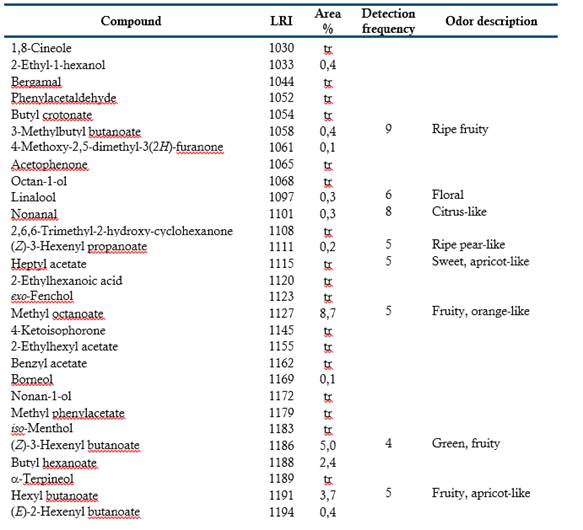
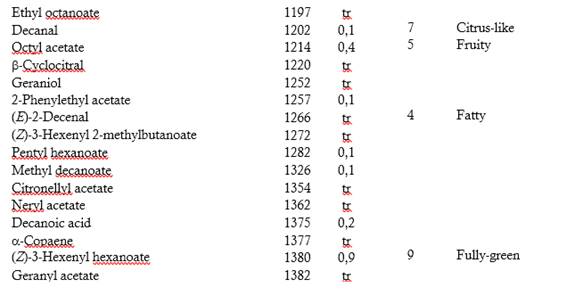
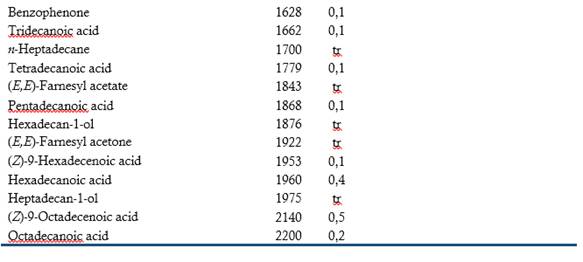
Table 1 shows the 128 volatile compounds isolated from sour guava fruit, representing 99 % of the isolated compounds, as well as the odor detected by sniffers. According to their chemical group, the volatile compounds are classified as 49 esters, 20 terpenes, 14 alcohols, 14 aldehydes, 13 acids, 7 ketones, 5 hydrocarbons, 3 oxides, 2 furans and one S-compound.
The semi-quantitative distribution of the fruit volatiles chemical families included esters (88.7 %), alcohols (3.8 %), acids (2.4 %), oxides (2.4 %), terpenes (0.9 %), aldehydes (0.7 %), ketones (0.5 %), hydrocarbons (0.1 %), furans (0.1 %) and S-compound (traces). Major components (> 8 %) were (Z)-3-hexenyl acetate, butyl butanoate, butyl acetate, methyl octanoate, methyl hexanoate and hexyl acetate. The GC-O was performed to categorize the volatile compounds according to their odor potency. Twenty-six compounds exhibited frequency factors ( 3 from a maximum of nine and therefore, they should contribute to the overall sour guava aroma. Of them, (Z)-3-hexenyl acetate (green, fruity), hexyl acetate (fruity, floral), 3-methylbutyl butanoate (ripe fruity) and (Z)-3-hexenyl hexanoate (fully green) were the most-odor active compounds. (Z)-3-Hexenyl acetate and hexyl acetate were among the most abundant in the composition and had high detection frequencies. It is interesting to note that 3-methylbutyl butanoate and (Z)-3-hexenyl hexanoate, which very low proportions also had high detection frequencies. Besides this, the relevance of aliphatic esters, particularly those related to C6 compounds, as odor-active compounds of sour guava fruit was tentatively demonstrated.
Esters seemed to be the important aroma compounds in fruits by their fruity notes. Some of them have been reported as key compounds in Psidium spp. fruits (Steinhaus et al., 2008, 2009; Pino & Bent, 2013; Buranelo-Egea et al., 2014; Cuadrado-Silva et al., 2017).
A quantitative approach based on absolute concentrations and the calculation of odor activity values combined with sensory studies needs to be done to determine the actual contribution of these volatile compounds to sour guava fruit, including model and omission sensory experiments.
Conclusions
A total of 128 volatile compounds were identified, for the first time, in sour guava fruit, including 49 esters, 20 terpenes, 14 alcohols, 14 aldehydes, 13 acids, 7 ketones, 5 hydrocarbons, 3 oxides, 2 furans and one S-compound. Twenty-six of them were considered as aroma-active compounds, from which the most important were (Z)-3-hexenyl acetate, hexyl acetate, 3-methylbutyl butanoate and (Z)-3-hexenyl hexanoate. The relevance of aliphatic esters, particularly those related to C6 compounds, as odor-active compounds of sour guava fruit was tentatively demonstrated.